Abstract
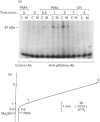
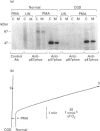
Activation of human neutrophil NADPH oxidase requires the interaction of cytosolic and membrane-associated components. Evidence has been accumulated that in phorbol 12-myristate 13-acetate (PMA)-stimulated neutrophils, the translocation to the plasma membrane of the cytosolic components p47phox and p67phox and the phosphorylation of p47phox are essential steps in activation of NADPH oxidase. No direct evidence has been presented to date as to whether p67phox is also phosphorylated. To address this problem we have immunoprecipitated p67phox from neutrophil cytosol and membrane fractions. The results indicate that, very soon after activation with PMA (20 s), p67phox was present in a phosphorylated form in the cytosol and in the membranes. At later times (1-3 min) the extent of p67phox phosphorylation continuously increased both in the cytosol and in the membrane fraction, while oxygen consumption reached the maximal rate within 40 s, and then remained linear. p67phox was also phosphorylated in formyl-methionyl-leucyl-phenylalanine-activated neutrophils. That the phosphorylated p67 protein we identified in immunoprecipitation experiments was p67phox was confirmed by the observation that no phosphorylated band of 67 kDa was immunoprecipitated from the cytosol and membranes of PMA-stimulated neutrophils from a p67phox-deficient chronic granulomatous disease patient. In this case, p47phox was normally phosphorylated. These data demonstrate that: (1) the phosphorylation of p67phox is correlated with activation of NADPH oxidase, and (2) continuous phosphorylation of p67phox is required in order to maintain the linearity of the respiratory burst.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Babior B. M. Endogenous protein phosphorylation by resting and activated human neutrophils. Blood. 1983 Feb;61(2):333–340. [PubMed] [Google Scholar]
- Bellavite P. The superoxide-forming enzymatic system of phagocytes. Free Radic Biol Med. 1988;4(4):225–261. doi: 10.1016/0891-5849(88)90044-5. [DOI] [PubMed] [Google Scholar]
- Ding J., Badwey J. A. Effects of antagonists of protein phosphatases on superoxide release by neutrophils. J Biol Chem. 1992 Mar 25;267(9):6442–6448. [PubMed] [Google Scholar]
- Dusi S., Della Bianca V., Grzeskowiak M., Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca(2+)-depleted human neutrophils. Biochem J. 1993 Feb 15;290(Pt 1):173–178. doi: 10.1042/bj2900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi S., Poli G., Berton G., Catalano P., Fornasa C. V., Peserico A. Chronic granulomatous disease in an adult female with granulomatous cheilitis. Evidence for an X-linked pattern of inheritance with extreme lyonization. Acta Haematol. 1990;84(1):49–56. doi: 10.1159/000205028. [DOI] [PubMed] [Google Scholar]
- Garcia R. C., Whitaker M., Heyworth P. G., Segal A. W. Okadaic acid produces changes in phosphorylation and translocation of proteins and in intracellular calcium in human neutrophils. Relationship with the activation of the NADPH oxidase by different stimuli. Biochem J. 1992 Sep 15;286(Pt 3):687–692. doi: 10.1042/bj2860687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., De Togni P., Papini E., Rossi F. Independence with respect to Ca2+ changes of the neutrophil respiratory and secretory response to exogenous phospholipase C and possible involvement of diacylglycerol and protein kinase C. Biochim Biophys Acta. 1985 Jan 18;844(1):81–90. doi: 10.1016/0167-4889(85)90237-x. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Badwey J. A. Continuous phosphorylation of both the 47 and the 49 kDa proteins occurs during superoxide production by neutrophils. Biochim Biophys Acta. 1990 May 2;1052(2):299–305. doi: 10.1016/0167-4889(90)90225-3. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Badwey J. A. Protein phosphorylation associated with the stimulation of neutrophils. Modulation of superoxide production by protein kinase C and calcium. J Bioenerg Biomembr. 1990 Feb;22(1):1–26. doi: 10.1007/BF00762842. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Segal A. W. Further evidence for the involvement of a phosphoprotein in the respiratory burst oxidase of human neutrophils. Biochem J. 1986 Nov 1;239(3):723–731. doi: 10.1042/bj2390723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Shrimpton C. F., Segal A. W. Localization of the 47 kDa phosphoprotein involved in the respiratory-burst NADPH oxidase of phagocytic cells. Biochem J. 1989 May 15;260(1):243–248. doi: 10.1042/bj2600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I. M., Verhoeven A. J., van der Bend R. L., Weening R. S., Roos D. Purified protein kinase C phosphorylates a 47-kDa protein in control neutrophil cytoplasts but not in neutrophil cytoplasts from patients with the autosomal form of chronic granulomatous disease. J Biol Chem. 1988 Feb 15;263(5):2352–2357. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Garrett M. C., Fujii H., Nunoi H. Characterization of neutrophil NADPH oxidase factors p47-phox and p67-phox from recombinant baculoviruses. J Biol Chem. 1991 Oct 15;266(29):19812–19818. [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Volpp B. D., McCormick S., Leidal K. G., Clark R. A. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991 Mar 25;266(9):5911–5917. [PubMed] [Google Scholar]
- Reibman J., Korchak H. M., Vosshall L. B., Haines K. A., Rich A. M., Weissmann G. Changes in diacylglycerol labeling, cell shape, and protein phosphorylation distinguish "triggering" from "activation" of human neutrophils. J Biol Chem. 1988 May 5;263(13):6322–6328. [PubMed] [Google Scholar]
- Rodaway A. R., Teahan C. G., Casimir C. M., Segal A. W., Bentley D. L. Characterization of the 47-kilodalton autosomal chronic granulomatous disease protein: tissue-specific expression and transcriptional control by retinoic acid. Mol Cell Biol. 1990 Oct;10(10):5388–5396. doi: 10.1128/mcb.10.10.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Kleinberg M. E., Nunoi H., Leto T., Gallin J. I., Malech H. L. Evidence for a functional cytoplasmic domain of phagocyte oxidase cytochrome b558. J Biol Chem. 1990 May 25;265(15):8745–8750. [PubMed] [Google Scholar]
- Rotrosen D., Leto T. L. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. Translocation to membrane is associated with distinct phosphorylation events. J Biol Chem. 1990 Nov 15;265(32):19910–19915. [PubMed] [Google Scholar]
- Schneider C., Zanetti M., Romeo D. Surface-reactive stimuli selectively increase protein phosphorylation in human neutrophils. FEBS Lett. 1981 May 5;127(1):4–8. doi: 10.1016/0014-5793(81)80327-4. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Curnutte J. T. Molecular basis of chronic granulomatous disease. Blood. 1991 Feb 15;77(4):673–686. [PubMed] [Google Scholar]