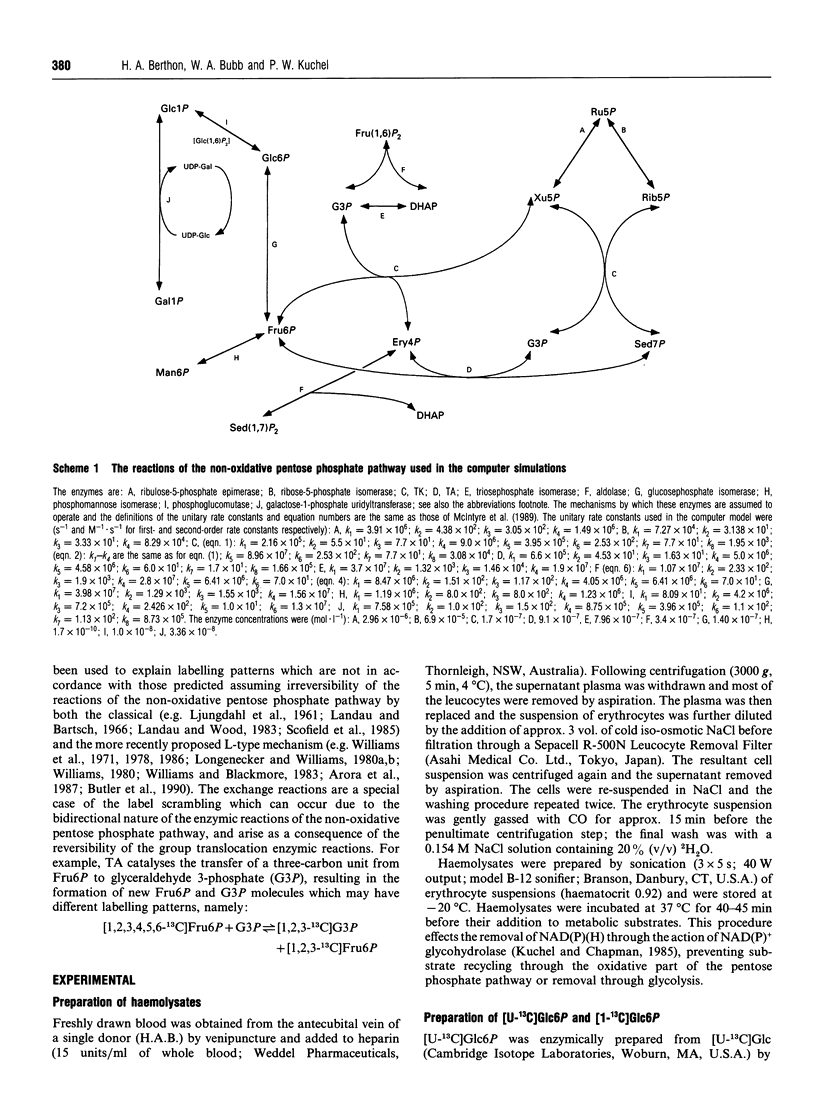
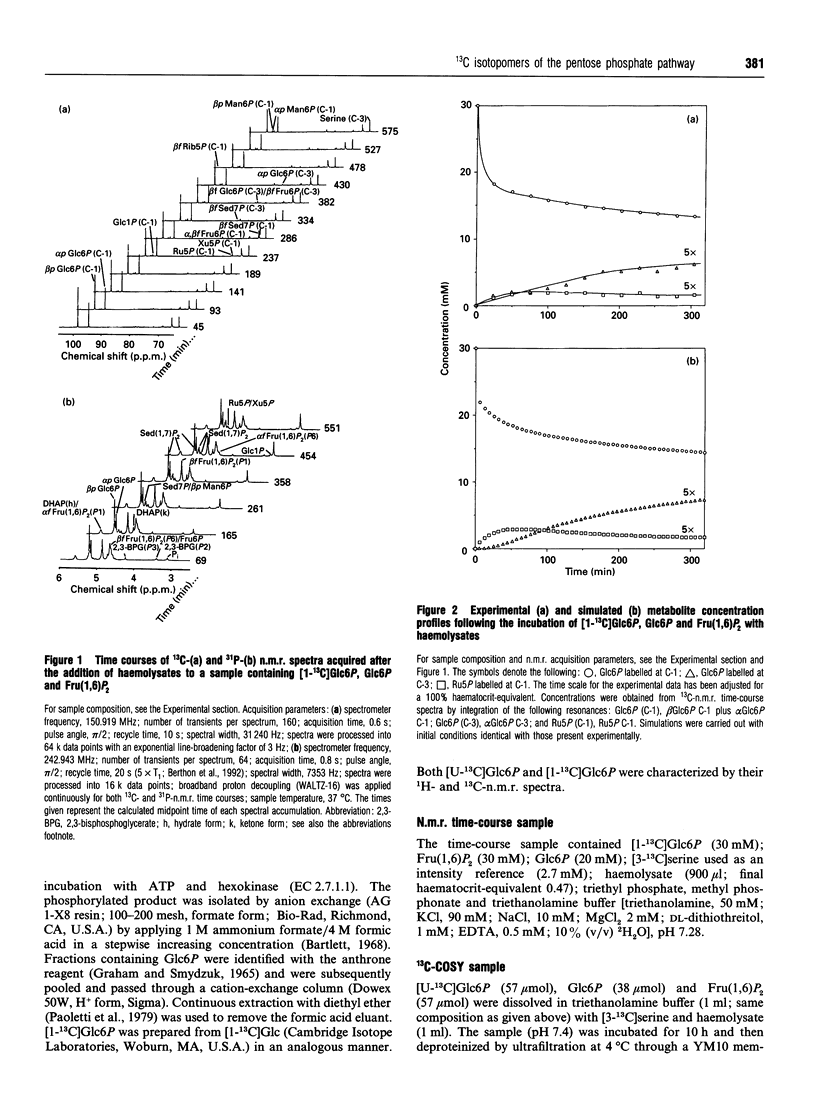
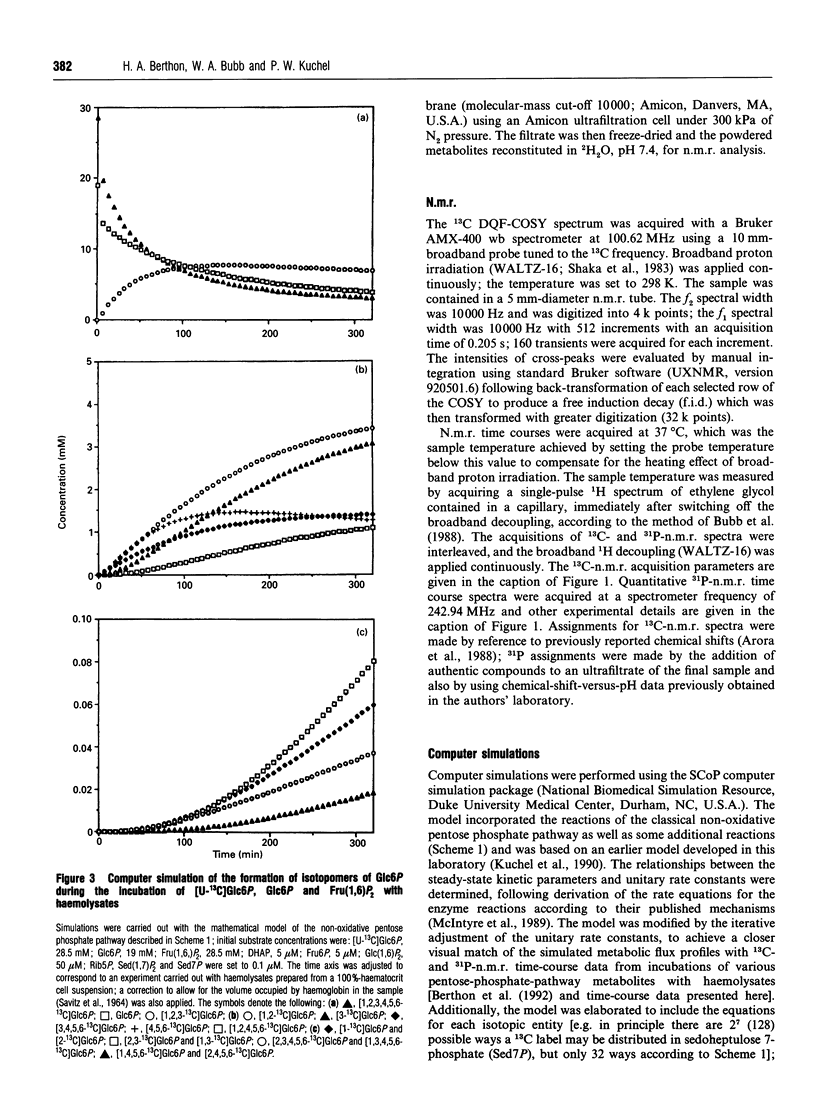
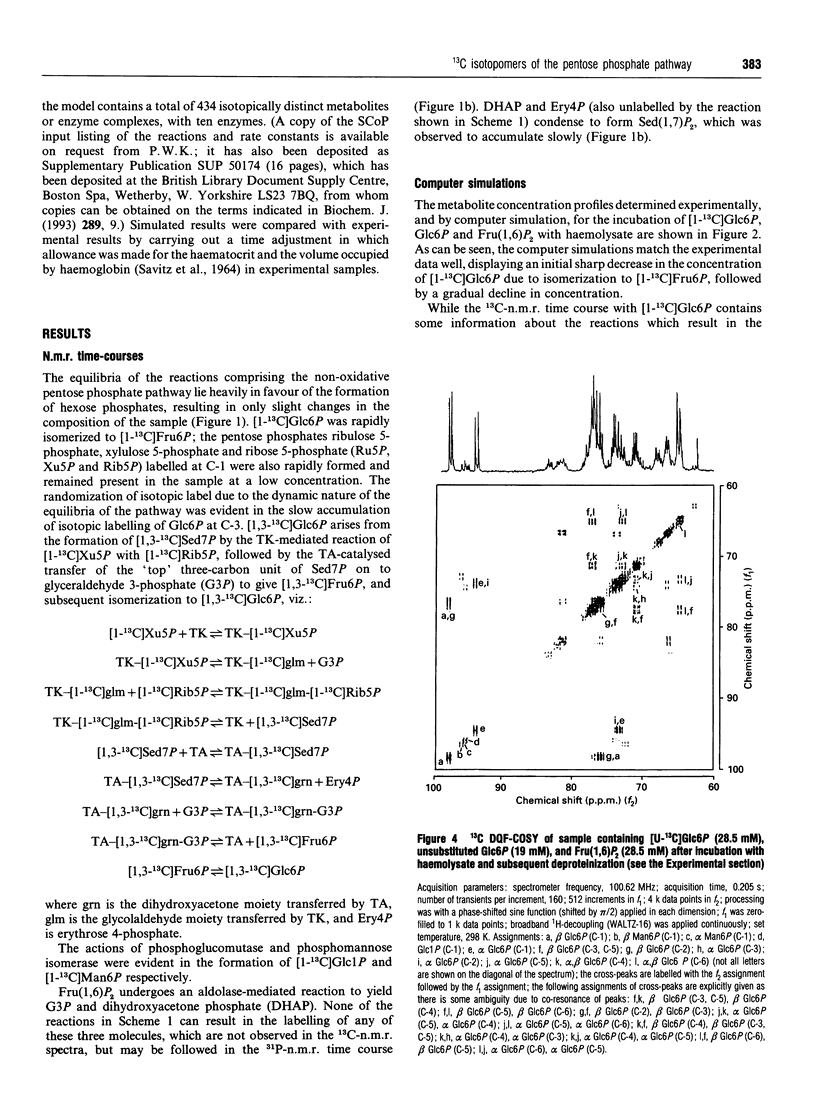
Abstract
13C double-quantum filtered correlation spectroscopy (DQF-COSY) provides a novel method for the detection of reactions involving carbon-bond scissions. We report the use of this technique to investigate isotopic exchange reactions of the non-oxidative pentose phosphate pathway in human erythrocytes. These exchange reactions resulted in the formation of a range of isotopic isomers (isotopomers) of glucose 6-phosphate after incubation of a mixture of universally 13C-labelled and unlabelled glucose 6-phosphate with fructose 1,6-bisphosphate and haemolysates. These isotopomers were detected in the coupling patterns of cross-peaks within the DQF-COSY spectrum of the deproteinized sample. A computer model which fully describes the reactions of the non-oxidative pentose phosphate pathway in human erythrocytes has previously been constructed and tested with 31P n.m.r. time-course data in our laboratory. This model was refined using 13C n.m.r. time-course data and extended to include the range of isotopomers which may be formed experimentally by the reactions of the non-oxidative pentose phosphate pathway. The isotopomer ratios obtained experimentally from the DQF-COSY spectrum were consistent with simulations generated by this model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora K. K., Collins J. G., MacLeod J. K., Williams J. F. Rapid methods for the high yield synthesis of carbon-13 enriched intermediates of the pentose-phosphate pathway. Biol Chem Hoppe Seyler. 1988 Jul;369(7):549–557. doi: 10.1515/bchm3.1988.369.2.549. [DOI] [PubMed] [Google Scholar]
- Arora K. K., Longenecker J. P., Williams J. F. Mechanism and quantitative contribution of the pentose pathway to the glucose metabolism of Morris hepatoma 5123C. Int J Biochem. 1987;19(2):133–146. doi: 10.1016/0020-711x(87)90324-7. [DOI] [PubMed] [Google Scholar]
- Bartlett G. R. Phosphorus compounds in the human erythrocyte. Biochim Biophys Acta. 1968 Mar 11;156(2):221–230. doi: 10.1016/0304-4165(68)90251-1. [DOI] [PubMed] [Google Scholar]
- Berthon H. A., Kuchel P. W., Nixon P. F. High control coefficient of transketolase in the nonoxidative pentose phosphate pathway of human erythrocytes: NMR, antibody, and computer simulation studies. Biochemistry. 1992 Dec 29;31(51):12792–12798. doi: 10.1021/bi00166a012. [DOI] [PubMed] [Google Scholar]
- Butler R. N., Arora K. K., Collins J. G., Flanigan I., Lawson M. J., Roberts-Thomson I. C., Williams J. F. Pentose phosphate pathway in rat colonic epithelium. Biochem Int. 1990 Oct;22(2):249–260. [PubMed] [Google Scholar]
- Cerdan S., Seelig J. NMR studies of metabolism. Annu Rev Biophys Biophys Chem. 1990;19:43–67. doi: 10.1146/annurev.bb.19.060190.000355. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Williams J. F., Blackmore P. F. The transketolase exchange reaction in vitro. Biochem J. 1971 Nov;125(1):381–384. doi: 10.1042/bj1250381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Rognstad R., Shulman R. G., Katz J. A comparison of 13C nuclear magnetic resonance and 14C tracer studies of hepatic metabolism. J Biol Chem. 1981 Apr 10;256(7):3428–3432. [PubMed] [Google Scholar]
- Crawford J. M., Blum J. J. Quantitative analysis of flux along the gluconeogenic, glycolytic and pentose phosphate pathways under reducing conditions in hepatocytes isolated from fed rats. Biochem J. 1983 Jun 15;212(3):585–598. doi: 10.1042/bj2120585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopher A., Vaisman N., Mandel H., Lapidot A. Determination of fructose metabolic pathways in normal and fructose-intolerant children: a 13C NMR study using [U-13C]fructose. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5449–5453. doi: 10.1073/pnas.87.14.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D., Smydzuk J. Use of anthrone in the quantitative determination of hexose phosphates. Anal Biochem. 1965 May;11(2):246–255. doi: 10.1016/0003-2697(65)90012-6. [DOI] [PubMed] [Google Scholar]
- Jeffrey F. M., Rajagopal A., Malloy C. R., Sherry A. D. 13C-NMR: a simple yet comprehensive method for analysis of intermediary metabolism. Trends Biochem Sci. 1991 Jan;16(1):5–10. doi: 10.1016/0968-0004(91)90004-f. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Gopher A., Lapidot A. Metabolic pathways leading to liver glycogen repletion in vivo, studied by GC-MS and NMR. FEBS Lett. 1986 Aug 11;204(1):29–32. doi: 10.1016/0014-5793(86)81381-3. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Korman S. H., Gutman A., Lapidot A. Estimation of glucose carbon recycling in children with glycogen storage disease: A 13C NMR study using [U-13C]glucose. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4690–4694. doi: 10.1073/pnas.86.12.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W., Berthon H. A., Bubb W. A., Bulliman B. T., Collins J. G. Computer simulation of the pentose-phosphate pathway and associated metabolism used in conjunction with NMR experimental data from human erythrocytes. Biomed Biochim Acta. 1990;49(8-9):757–770. [PubMed] [Google Scholar]
- Kuchel P. W., Chapman B. E. Direct observation of the NAD glycohydrolase reaction in human erythrocytes using NMR spectroscopy. Experientia. 1985 Jan 15;41(1):53–55. doi: 10.1007/BF02005870. [DOI] [PubMed] [Google Scholar]
- LJUNGDAHL L., WOOD H. G., RACKER E., COURI D. Formation of unequally labeled fructose 6-phosphate by an exchange reaction catalyzed by transaldolase. J Biol Chem. 1961 Jun;236:1622–1625. [PubMed] [Google Scholar]
- Landau B. R., Bartsch G. E. Estimations of pathway contributions to glucose metabolism and the transaldolase reactions. J Biol Chem. 1966 Feb 10;241(3):741–749. [PubMed] [Google Scholar]
- Longenecker J. P., Williams J. F. Quantitative measurement of the L-type pentose phosphate cycle with [2-14C]glucose and [5-14C]glucose in isolated hepatocytes. Biochem J. 1980 Jun 15;188(3):859–865. doi: 10.1042/bj1880859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker J. P., Williams J. F. Use of [2-14C]glucose and [5-14C]glucose for evaluating the mechanism and quantitative significance of the 'liver-cell' pentose cycle. Biochem J. 1980 Jun 15;188(3):847–857. doi: 10.1042/bj1880847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy C. R., Sherry A. D., Jeffrey F. M. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am J Physiol. 1990 Sep;259(3 Pt 2):H987–H995. doi: 10.1152/ajpheart.1990.259.3.H987. [DOI] [PubMed] [Google Scholar]
- Malloy C. R., Sherry A. D., Jeffrey F. M. Carbon flux through citric acid cycle pathways in perfused heart by 13C NMR spectroscopy. FEBS Lett. 1987 Feb 9;212(1):58–62. doi: 10.1016/0014-5793(87)81556-9. [DOI] [PubMed] [Google Scholar]
- McIntyre L. M., Thorburn D. R., Bubb W. A., Kuchel P. W. Comparison of computer simulations of the F-type and L-type non-oxidative hexose monophosphate shunts with 31P-NMR experimental data from human erythrocytes. Eur J Biochem. 1989 Mar 15;180(2):399–420. doi: 10.1111/j.1432-1033.1989.tb14662.x. [DOI] [PubMed] [Google Scholar]
- Paoletti F., Williams J. F., Horecker B. L. Synthesis and cleavage of octulose bisphosphates with liver and muscle aldolases. Arch Biochem Biophys. 1979 Dec;198(2):614–619. doi: 10.1016/0003-9861(79)90538-1. [DOI] [PubMed] [Google Scholar]
- SAVITZ D., SIDEL V. W., SOLOMON A. K. OSMOTIC PROPERTIES OF HUMAN RED CELLS. J Gen Physiol. 1964 Sep;48:79–94. doi: 10.1085/jgp.48.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield R. F., Kosugi K., Chandramouli V., Kumaran K., Schumann W. C., Landau B. R. The nature of the pentose pathway in liver. J Biol Chem. 1985 Dec 15;260(29):15439–15444. [PubMed] [Google Scholar]
- Scott A. I., Baxter R. L. Applications of 13C NMR to metabolic studies. Annu Rev Biophys Bioeng. 1981;10:151–174. doi: 10.1146/annurev.bb.10.060181.001055. [DOI] [PubMed] [Google Scholar]
- Sherry A. D., Nunnally R. L., Peshock R. M. Metabolic studies of pyruvate- and lactate-perfused guinea pig hearts by 13C NMR. Determination of substrate preference by glutamate isotopomer distribution. J Biol Chem. 1985 Aug 5;260(16):9272–9279. [PubMed] [Google Scholar]
- Walker T. E., Han C. H., Kollman V. H., London R. E., Matwiyoff N. A. 13C nuclear magnetic resonance studies of the biosynthesis by Microbacterium ammoniaphilum of L-glutamate selectively enriched with carbon-13. J Biol Chem. 1982 Feb 10;257(3):1189–1195. [PubMed] [Google Scholar]
- Williams J. F., Arora K. K., Longenecker J. P. The pentose pathway: a random harvest. Impediments which oppose acceptance of the classical (F-type) pentose cycle for liver, some neoplasms and photosynthetic tissue. The case for the L-type pentose pathway. Int J Biochem. 1987;19(9):749–817. doi: 10.1016/0020-711x(87)90239-4. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Blackmore P. F., Clark M. G. New reaction sequences for the non-oxidative pentose phosphate pathway. Biochem J. 1978 Oct 15;176(1):257–282. doi: 10.1042/bj1760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Blackmore P. F. Non-oxidative synthesis of pentose 5-phosphate from hexose 6-phosphate and triose phosphate by the L-type pentose pathway. Int J Biochem. 1983;15(6):797–816. doi: 10.1016/0020-711x(83)90152-0. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Gordon R. D., Gerdes R. G., Rienits K. G., Arora K. K., Anderson J. The metabolic significance of pentose cycle measurements in perfused liver. Biochem Int. 1986 Aug;13(2):321–333. [PubMed] [Google Scholar]
- Williams J. F., Rienits K. G., Schofield P. J., Clark M. G. The pentose phosphate pathway in rabbit liver. Studies on the metabolic sequence and quantitative role of the pentose phosphate cycle by using a system in situ. Biochem J. 1971 Aug;123(5):923–943. doi: 10.1042/bj1230923. [DOI] [PMC free article] [PubMed] [Google Scholar]


