Abstract
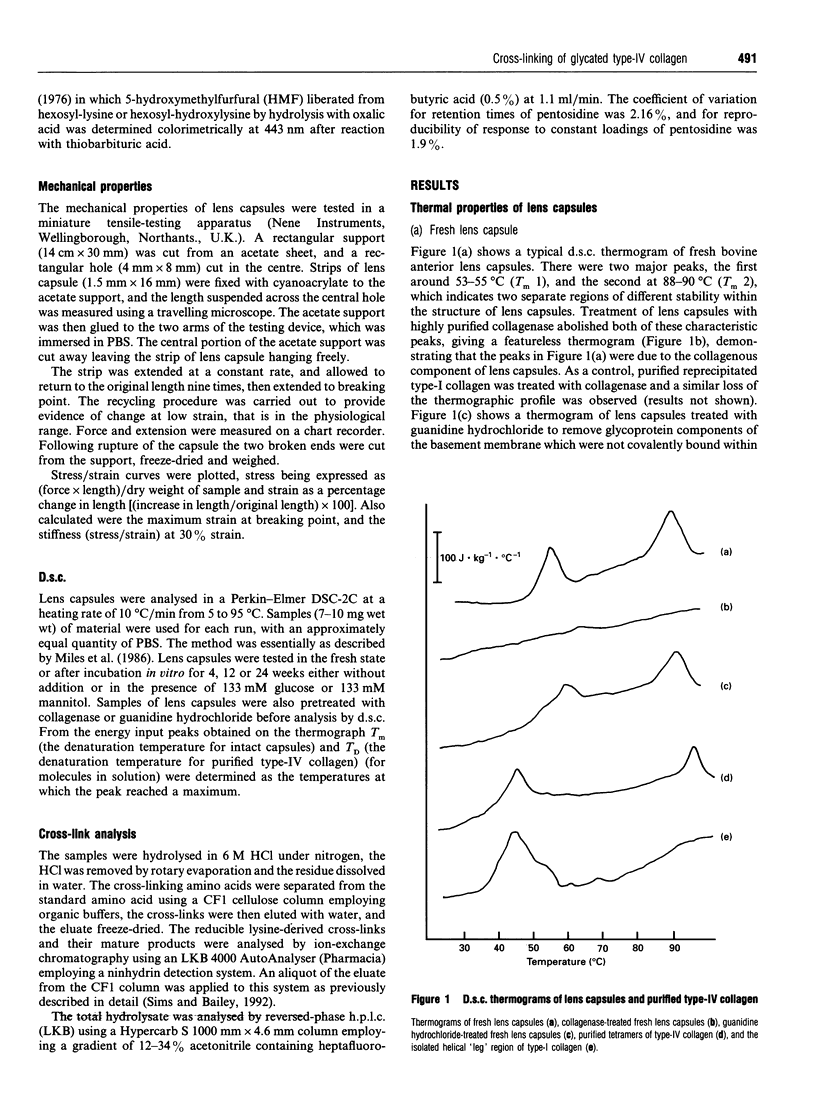
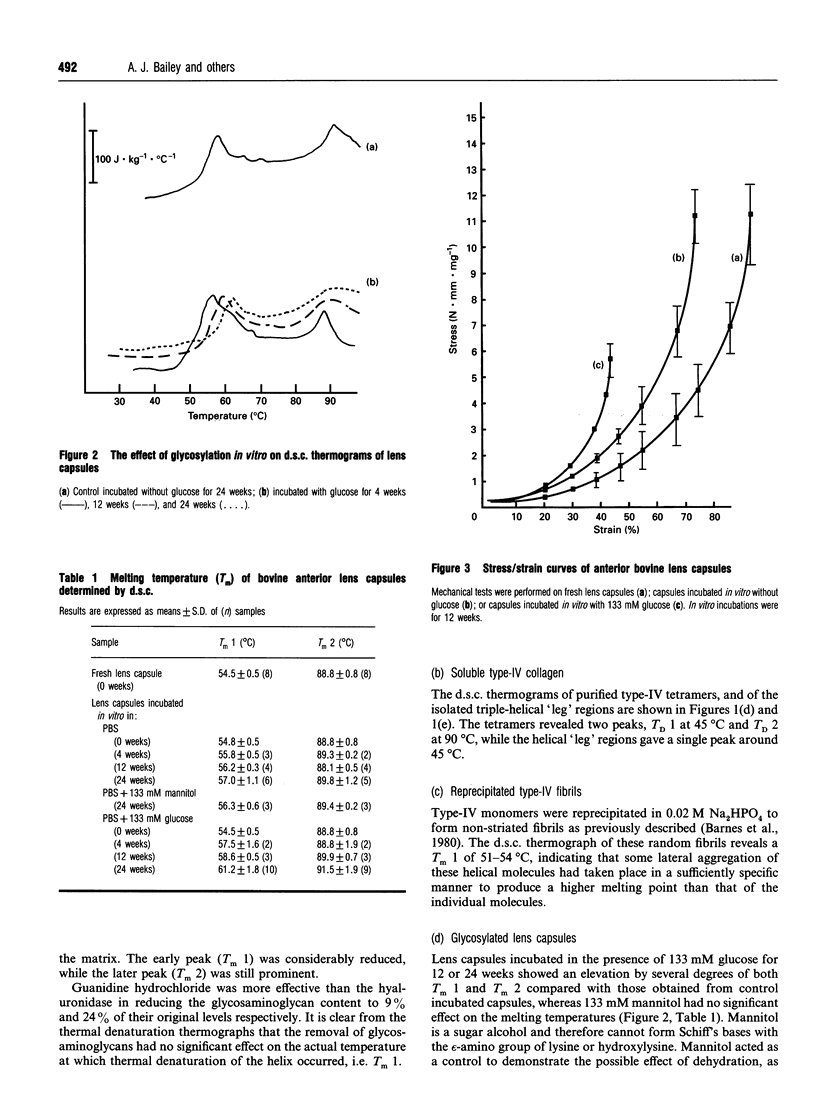
The incubation of lens capsules with glucose in vitro resulted in changes in the mechanical and thermal properties of type-IV collagen consistent with increased cross-linking. Differential scanning calorimetry (d.s.c.) of fresh lens capsules showed two major peaks at melting temperatures Tm 1 and Tm 2 at approx. 54 degrees C and 90 degrees C, which can be attributed to the denaturation of the triple helix and 7S domains respectively. Glycosylation of lens capsules in vitro for 24 weeks caused an increase in Tm 1 from 54 degrees C to 61 degrees C, while non-glycosylated, control incubated capsules increased to a Tm 1 of 57 degrees C. The higher temperature required to denature the type-IV collagen after incubation in vitro suggested increased intermolecular cross-linking. Glycosylated lens capsules were more brittle than fresh samples, breaking at a maximum strain of 36.8 +/- 1.8% compared with 75.6 +/- 6.3% for the fresh samples. The stress at maximum strain (or 'strength') was dramatically reduced from 12.0 to 4.7 N.mm.mg-1 after glycosylation in vitro. The increased constraints within the system leading to loss of strength and increased brittleness suggested not only the presence of more cross-links but a difference in the location of these cross-links compared with the natural lysyl-aldehyde-derived cross-links. The chemical nature of the fluorescent glucose-derived cross-link following glycosylation was determined as pentosidine, at a concentration of 1 pentosidine molecule per 600 collagen molecules after 24 weeks incubation. Pentosidine was also determined in the lens capsules obtained from uncontrolled diabetics at a level of about 1 per 100 collagen molecules. The concentration of these pentosidine cross-links is far too small to account for the observed changes in the thermal and mechanical properties following incubation in vitro, clearly indicating that another as yet undefined, but apparently more important cross-linking mechanism mediated by glucose is taking place.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J., Light N. Cross-linking in type IV collagen. Biochem J. 1984 Mar 15;218(3):713–723. doi: 10.1042/bj2180713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard K., Gathercole L. J., Bailey A. J. Basement membrane collagen--evidence for a novel molecular packing. FEBS Lett. 1987 Feb 9;212(1):49–52. doi: 10.1016/0014-5793(87)81554-5. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Bailey A. J., Gordon J. L., MacIntyre D. E. Platelet aggregaton by basement membrane-associated collagens. Thromb Res. 1980 May 1;18(3-4):375–388. doi: 10.1016/0049-3848(80)90333-3. [DOI] [PubMed] [Google Scholar]
- Brennan M. Changes in solubility, non-enzymatic glycation, and fluorescence of collagen in tail tendons from diabetic rats. J Biol Chem. 1989 Dec 15;264(35):20947–20952. [PubMed] [Google Scholar]
- Brown D. M., Klein D. J., Michael A. F., Oegema T. R. 35S-glycosaminoglycan and 35S-glycopeptide metabolism by diabetic glomeruli and aorta. Diabetes. 1982 May;31(5 Pt 1):418–425. doi: 10.2337/diab.31.5.418. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Ulrich P. C., Bucala R., Cerami A. Detection of an advanced glycosylation product bound to protein in situ. J Biol Chem. 1985 Jul 5;260(13):7970–7974. [PubMed] [Google Scholar]
- Cohen M. P., Urdanivia E., Surma M., Wu V. Y. Increased glycosylation of glomerular basement membrane collagen in diabetes. Biochem Biophys Res Commun. 1980 Jul 31;95(2):765–769. doi: 10.1016/0006-291x(80)90852-9. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Fietzek P. P., Allmann H., Rauterberg J., Wachter E. Ordering of cyanogen bromide peptides of type III collagen based on their homology to type I collagen: preservation of sites for crosslink formation during evolution. Proc Natl Acad Sci U S A. 1977 Jan;74(1):84–86. doi: 10.1073/pnas.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. F., Wakely J. The elastic constants and ultrastructural organization of a basement membrane (lens capsule). Proc R Soc Lond B Biol Sci. 1976 Jun 30;193(1113):335–358. doi: 10.1098/rspb.1976.0051. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Fu M. X., Knecht K. J., Thorpe S. R., Baynes J. W. Role of oxygen in cross-linking and chemical modification of collagen by glucose. Diabetes. 1992 Oct;41 (Suppl 2):42–48. doi: 10.2337/diab.41.2.s42. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Bunn H. F., Spiro R. G. Nonenzymatic glycation of basement membranes from human glomeruli and bovine sources. Effect of diabetes and age. Diabetes. 1988 Aug;37(8):1144–1150. doi: 10.2337/diab.37.8.1144. [DOI] [PubMed] [Google Scholar]
- Gelman R. A., Blackwell J., Kefalides N. A., Tomichek E. Thermal stability of basement membrane collagen. Biochim Biophys Acta. 1976 Apr 14;427(2):492–496. doi: 10.1016/0005-2795(76)90191-4. [DOI] [PubMed] [Google Scholar]
- Grandhee S. K., Monnier V. M. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991 Jun 25;266(18):11649–11653. [PubMed] [Google Scholar]
- Heathcote J. G., Bailey A. J., Grant M. E. Studies on the assembly of the rat lens capsule. Biosynthesis of a cross-linked collagenous component of high molecular weight. Biochem J. 1980 Aug 15;190(2):229–237. doi: 10.1042/bj1900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M. J., Light N. D., Bailey A. J. Evidence for glucose-mediated covalent cross-linking of collagen after glycosylation in vitro. Biochem J. 1985 Feb 1;225(3):745–752. doi: 10.1042/bj2250745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn R. R., Cerami A., Monnier V. M. Collagen aging in vitro by nonenzymatic glycosylation and browning. Diabetes. 1984 Jan;33(1):57–59. doi: 10.2337/diab.33.1.57. [DOI] [PubMed] [Google Scholar]
- Le Pape A., Muh J. P., Bailey A. J. Characterization of N-glycosylated type I collagen in streptozotocin-induced diabetes. Biochem J. 1981 Aug 1;197(2):405–412. doi: 10.1042/bj1970405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer T. F., Gibney E., Fitch J. M., Gross J., Mayne R. Thermal stability of the helical structure of type IV collagen within basement membranes in situ: determination with a conformation-dependent monoclonal antibody. J Cell Biol. 1984 Oct;99(4 Pt 1):1405–1409. doi: 10.1083/jcb.99.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S. S., Shin D. H., Newman B. L., Lee J. H., Lupovitch A., Drakes G. H. Glycosylation in vivo of human lens capsule (basement membrane) and diabetes mellitus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):51–56. doi: 10.1016/0006-291x(83)91539-5. [DOI] [PubMed] [Google Scholar]
- Miles C. A. Kinetics of collagen denaturation in mammalian lens capsules studied by differential scanning calorimetry. Int J Biol Macromol. 1993 Oct;15(5):265–271. doi: 10.1016/0141-8130(93)90025-h. [DOI] [PubMed] [Google Scholar]
- Miles C. A., Mackey B. M., Parsons S. E. Differential scanning calorimetry of bacteria. J Gen Microbiol. 1986 Apr;132(4):939–952. doi: 10.1099/00221287-132-4-939. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Stevens V. J., Cerami A. Nonenzymatic glycosylation, sulfhydryl oxidation, and aggregation of lens proteins in experimental sugar cataracts. J Exp Med. 1979 Nov 1;150(5):1098–1107. doi: 10.1084/jem.150.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge F. G., Fernandes A. A., Monnier V. M. Mechanism of formation of the putative advanced glycosylation end product and protein cross-link 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole. J Biol Chem. 1988 Aug 5;263(22):10646–10652. [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Effect of diabetes on the glycosaminoglycan component of the human glomerular basement membrane. Diabetes. 1982 Aug;31(8 Pt 1):738–741. doi: 10.2337/diab.31.8.738. [DOI] [PubMed] [Google Scholar]
- Pongor S., Ulrich P. C., Bencsath F. A., Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci U S A. 1984 May;81(9):2684–2688. doi: 10.1073/pnas.81.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. K., Hudson B. G., Bailey A. J., Noelken M. E. Reductive cleavage of the disulfide bonds of the collagen IV noncollagenous domain in aqueous sodium dodecyl sulfate: absence of intermolecular nondisulfide cross-links. Biochem Biophys Res Commun. 1993 Jan 15;190(1):277–282. doi: 10.1006/bbrc.1993.1042. [DOI] [PubMed] [Google Scholar]
- Reiser K. M. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1991 Jan;196(1):17–29. doi: 10.3181/00379727-196-43158c. [DOI] [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem Biophys Res Commun. 1972 Jul 11;48(1):76–84. doi: 10.1016/0006-291x(72)90346-4. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Some observations on the ageing in vitro of reprecipitated collagen fibres. Biochim Biophys Acta. 1977 Jun 24;492(2):408–414. doi: 10.1016/0005-2795(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Rohrbach D. H., Hassell J. R., Kleinman H. K., Martin G. R. Alterations in the basement membrane (heparan sulfate) proteoglycan in diabetic mice. Diabetes. 1982 Feb;31(2):185–188. doi: 10.2337/diab.31.2.185. [DOI] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980 Nov;66(5):1179–1181. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Siebold B., Qian R. A., Glanville R. W., Hofmann H., Deutzmann R., Kühn K. Construction of a model for the aggregation and cross-linking region (7S domain) of type IV collagen based upon an evaluation of the primary structure of the alpha 1 and alpha 2 chains in this region. Eur J Biochem. 1987 Nov 2;168(3):569–575. doi: 10.1111/j.1432-1033.1987.tb13455.x. [DOI] [PubMed] [Google Scholar]
- Sims T. J., Bailey A. J. Quantitative analysis of collagen and elastin cross-links using a single-column system. J Chromatogr. 1992 Nov 6;582(1-2):49–55. doi: 10.1016/0378-4347(92)80301-6. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Search for a biochemical basis of diabetic microangiopathy. Diabetologia. 1976 Mar;12(1):1–14. doi: 10.1007/BF01221959. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ruiz J. M., López-Lacomba J. L., Cortijo M., Mateo P. L. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry. 1988 Mar 8;27(5):1648–1652. doi: 10.1021/bi00405a039. [DOI] [PubMed] [Google Scholar]
- Tarsio J. F., Reger L. A., Furcht L. T. Decreased interaction of fibronectin, type IV collagen, and heparin due to nonenzymatic glycation. Implications for diabetes mellitus. Biochemistry. 1987 Feb 24;26(4):1014–1020. doi: 10.1021/bi00378a006. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Trüeb B., Flückiger R., Winterhalter K. H. Nonenzymatic glycosylation of basement membrane collagen in diabetes mellitus. Coll Relat Res. 1984 Aug;4(4):239–251. doi: 10.1016/s0174-173x(84)80032-1. [DOI] [PubMed] [Google Scholar]
- Tsilibary E. C., Charonis A. S., Reger L. A., Wohlhueter R. M., Furcht L. T. The effect of nonenzymatic glucosylation on the binding of the main noncollagenous NC1 domain to type IV collagen. J Biol Chem. 1988 Mar 25;263(9):4302–4308. [PubMed] [Google Scholar]
- Viidik A. Functional properties of collagenous tissues. Int Rev Connect Tissue Res. 1973;6:127–215. doi: 10.1016/b978-0-12-363706-2.50010-6. [DOI] [PubMed] [Google Scholar]
- Walton H. A., Byrne J., Robinson G. B. Studies of the permeation properties of glomerular basement membrane: cross-linking renders glomerular basement membrane permeable to protein. Biochim Biophys Acta. 1992 Mar 20;1138(3):173–183. doi: 10.1016/0925-4439(92)90035-l. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Thorpe S. R., Baynes J. W. Glycation of amino groups in protein. Studies on the specificity of modification of RNase by glucose. J Biol Chem. 1985 Sep 5;260(19):10629–10636. [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Handelsman D. J., Delbridge L., Reeve T., Turtle J. R. The effect of salicylates on nonenzymatic glycosylation and thermal stability of collagen in diabetic rats. Diabetes. 1984 Aug;33(8):745–751. doi: 10.2337/diab.33.8.745. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Tsilibary E. C., Charonis A. S., Furthmayr H. Models for the self-assembly of basement membrane. J Histochem Cytochem. 1986 Jan;34(1):93–102. doi: 10.1177/34.1.3510247. [DOI] [PubMed] [Google Scholar]
- le Pape A., Guitton J. D., Muh J. P. Modification of glomerular basement membrane cross-links in experimental diabetic rats. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1214–1221. doi: 10.1016/0006-291x(81)91953-7. [DOI] [PubMed] [Google Scholar]


