Abstract
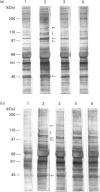
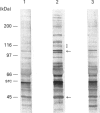
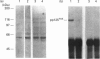
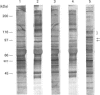
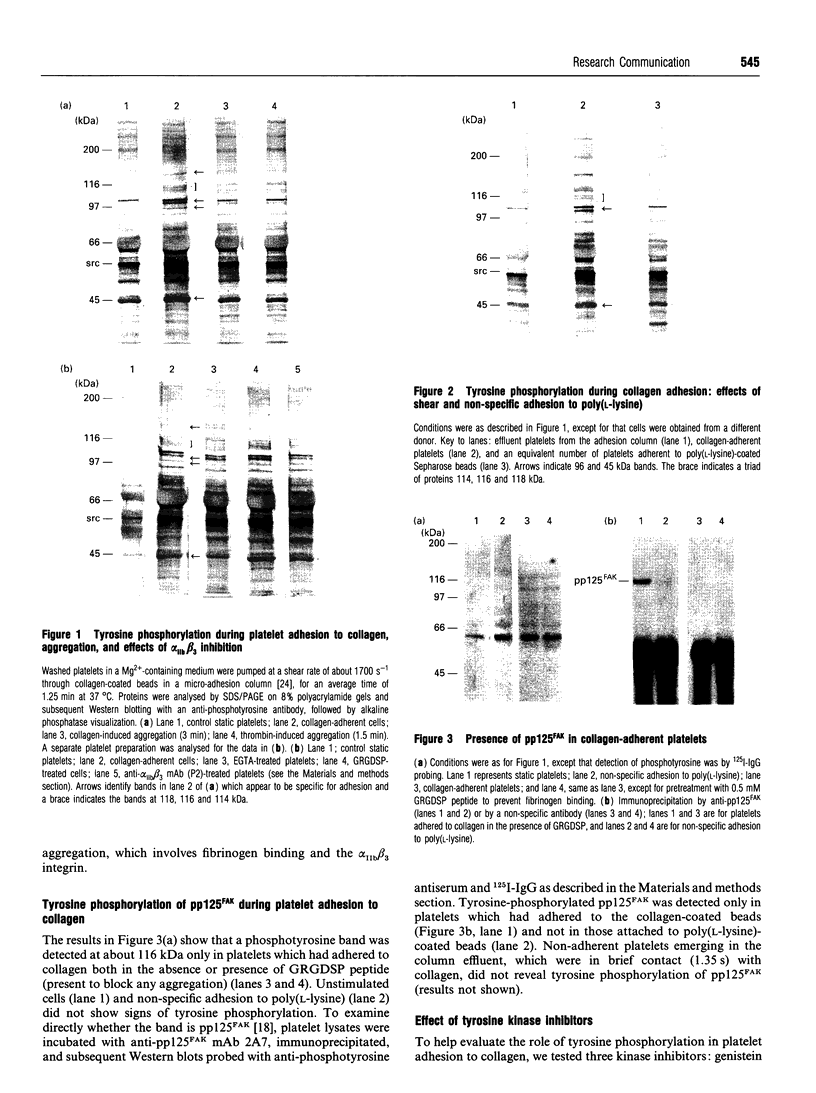
Adhesion of human platelets to collagen under arterial flow conditions mediated by the alpha 2 beta 1 integrin increased tyrosine phosphorylation of several proteins, one of which was the focal adhesion tyrosine kinase, pp125FAK. Tyrosine phosphorylation of pp125FAK did not occur in non-adherent flowing platelets or in platelets attached to poly(L-lysine). Neither adhesion nor tyrosine phosphorylation was affected by pretreatment of platelets with GRGDSP peptide or by anti-alpha IIb beta 3 monoclonal antibody P2. Adherent platelets retained their discoid shape, suggesting that induction of pp125FAK precedes platelet spreading. The tyrosine kinase inhibitor erbstatin decreased tyrosine phosphorylation in non-stimulated platelets and blocked platelet adhesion. These results suggest that pp125FAK plays an important role in platelet adhesion to collagen via the alpha 2 beta 1 integrin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bishop W. R., Petrin J., Wang L., Ramesh U., Doll R. J. Inhibition of protein kinase C by the tyrosine kinase inhibitor erbstatin. Biochem Pharmacol. 1990 Nov 1;40(9):2129–2135. doi: 10.1016/0006-2952(90)90245-g. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Pinault F. M. Activation of membrane protein-tyrosine phosphatase involving cAMP- and Ca2+/phospholipid-dependent protein kinases. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6696–6700. doi: 10.1073/pnas.88.15.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Dhar A., Paul A. K., Shukla S. D. Platelet-activating factor stimulation of tyrosine kinase and its relationship to phospholipase C in rabbit platelets: studies with genistein and monoclonal antibody to phosphotyrosine. Mol Pharmacol. 1990 Apr;37(4):519–525. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudette D. C., Holub B. J. Effect of genistein, a tyrosine kinase inhibitor, on U46619-induced phosphoinositide phosphorylation in human platelets. Biochem Biophys Res Commun. 1990 Jul 16;170(1):238–242. doi: 10.1016/0006-291x(90)91265-t. [DOI] [PubMed] [Google Scholar]
- Gear A. R. Rapid platelet morphological changes visualized by scanning-electron microscopy: kinetics derived from a quenched-flow approach. Br J Haematol. 1984 Mar;56(3):387–398. doi: 10.1111/j.1365-2141.1984.tb03969.x. [DOI] [PubMed] [Google Scholar]
- Gear A. R. Rapid reactions of platelets studied by a quenched-flow approach: aggregation kinetics. J Lab Clin Med. 1982 Dec;100(6):866–886. [PubMed] [Google Scholar]
- Golden A., Brugge J. S., Shattil S. J. Role of platelet membrane glycoprotein IIb-IIIa in agonist-induced tyrosine phosphorylation of platelet proteins. J Cell Biol. 1990 Dec;111(6 Pt 2):3117–3127. doi: 10.1083/jcb.111.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Lipfert L., Brugge J. S., Shattil S. J. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem. 1993 Jul 25;268(21):15868–15877. [PubMed] [Google Scholar]
- Hamawy M. M., Mergenhagen S. E., Siraganian R. P. Tyrosine phosphorylation of pp125FAK by the aggregation of high affinity immunoglobulin E receptors requires cell adherence. J Biol Chem. 1993 Apr 5;268(10):6851–6854. [PubMed] [Google Scholar]
- Horak I. D., Corcoran M. L., Thompson P. A., Wahl L. M., Bolen J. B. Expression of p60fyn in human platelets. Oncogene. 1990 Apr;5(4):597–602. [PubMed] [Google Scholar]
- Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. M., Lipfert L., Cunningham M., Brugge J. S., Ginsberg M. H., Shattil S. J. Adhesive ligand binding to integrin alpha IIb beta 3 stimulates tyrosine phosphorylation of novel protein substrates before phosphorylation of pp125FAK. J Cell Biol. 1993 Jul;122(2):473–483. doi: 10.1083/jcb.122.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Kunicki T. J., Nugent D. J., Staats S. J., Orchekowski R. P., Wayner E. A., Carter W. G. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J Biol Chem. 1988 Apr 5;263(10):4516–4519. [PubMed] [Google Scholar]
- Lipfert L., Haimovich B., Schaller M. D., Cobb B. S., Parsons J. T., Brugge J. S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992 Nov;119(4):905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Yamamura H. Thrombin and collagen induce rapid phosphorylation of a common set of cellular proteins on tyrosine in human platelets. J Biol Chem. 1989 May 5;264(13):7089–7091. [PubMed] [Google Scholar]
- Pelletier A. J., Bodary S. C., Levinson A. D. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol Biol Cell. 1992 Sep;3(9):989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanowska-Grabowska R., Gear A. R. High-speed platelet adhesion under conditions of rapid flow. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5754–5758. doi: 10.1073/pnas.89.13.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F., Eldor A., Grelac F., Bachelot C., Gazit A., Gilon C., Levy-Toledano S., Levitzki A. Inhibition of platelet activation by tyrosine kinase inhibitors. Biochem Pharmacol. 1992 Sep 1;44(5):881–888. doi: 10.1016/0006-2952(92)90119-4. [DOI] [PubMed] [Google Scholar]
- Salari H., Duronio V., Howard S. L., Demos M., Jones K., Reany A., Hudson A. T., Pelech S. L. Erbstatin blocks platelet activating factor-induced protein-tyrosine phosphorylation, polyphosphoinositide hydrolysis, protein kinase C activation, serotonin secretion and aggregation of rabbit platelets. FEBS Lett. 1990 Apr 9;263(1):104–108. doi: 10.1016/0014-5793(90)80715-u. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Molecular basis of platelet adhesion to collagen. Prog Clin Biol Res. 1988;283:291–314. [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. Transmembrane signalling by integrins. Trends Cell Biol. 1992 Oct;2(10):304–308. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Brugge J. S. Protein tyrosine phosphorylation and the adhesive functions of platelets. Curr Opin Cell Biol. 1991 Oct;3(5):869–879. doi: 10.1016/0955-0674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Protein kinase C involvement in focal adhesion formation. J Cell Sci. 1992 Feb;101(Pt 2):277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]