Abstract
We provide an update regarding the differences between men and women in short-term postoperative mortality after coronary artery bypass grafting (CABG) and highlight the differences in postoperative risk of stroke, myocardial infarction, and new onset atrial fibrillation. We included 23 studies, with a total of 3,971,267 patients (70.7% men, 29.3% women), and provided results for groups of unbalanced studies and propensity matched studies. For short-term mortality, the pooled odds ratio (OR) from unbalanced studies was 1.71 (with 95% CI 1.69–1.74, I2 = 0%, p = 0.7), and from propensity matched studies was 1.32 (95% CI 1.14–1.52, I2 = 76%, p < 0.01). For postoperative stroke, the pooled effects were OR = 1.50 (95% CI 1.35–1.66, I2 = 83%, p < 0.01) and OR = 1.31 (95% CI 1.02–1.67, I2 = 81%, p < 0.01). For myocardial infarction, the pooled effects were OR = 1.09 (95% CI = 0.78–1.53, I2 = 70%, p < 0.01) and OR = 1.03 (95% CI = 0.86–1.24, I2 = 43%, p = 0.18). For postoperative atrial fibrillation, the pooled effect from unbalanced studies was OR = 0.89 (95% CI = 0.82–0.96, I2 = 34%, p = 0.18). The short-term mortality risk after CABG is higher in women, compared to men. Women are at higher risk of postoperative stroke. There is no significant difference in the likelihood of postoperative myocardial infarction in women compared to men. Men are at higher risk of postoperative atrial fibrillation after CABG.
Keywords: Coronary artery bypass graft, Stroke, Myocardial infarction, Atrial fibrillation, Mortality, Meta-analysis
Subject terms: Cardiology, Diseases, Medical research, Risk factors
Introduction
Coronary artery bypass grafting (CABG) is the most frequently performed cardiac surgery worldwide1. Recent large-scale analyses have shown that after CABG, women are persistently at greater risk of operative death2–5. Although this disparity is generally attributed to preoperative and intraoperative differences between men and women, as well as to differences in biology and pathophysiology between the two sexes, its root causes have not yet been elucidated5. Considering women who undergo CABG are generally older, they also present higher comorbidities burden at the time of CABG, compared to men2,3. The meta-analysis of Bryce Robinson et al.2 focused on describing the differences between men and women following isolated CABG, by pooling results concerning operative mortality, late mortality, long-term myocardial infarction, stroke, repeat revascularization, and major adverse cardiac events (MACE). The authors concluded that females undergoing isolated CABG are not only at higher risk for operative and late mortality when compared with males, but also at higher risk of late non-fatal events including MACE, myocardial infarction, and stroke. Meta-regression analysis showed that the rate of preoperative myocardial infarction, smoking, renal failure and the number of grafts performed were associated with the operative mortality. In a meta-analysis which included 112 studies reporting unadjusted short-term mortality odds ratios and 25 studies which adjusted for various confounders, Shi et al.3 found a higher risk for death within 30-day after CABG in women, compared to men. Furthermore, to evaluate whether unmeasured confounding explains higher mortality, the authors performed a bias analysis simulation which considered the prevalence of prior cerebrovascular accident, heart failure, diabetes, and peripheral vascular disease. Under the assumption that the confounding effects on the risk of death were the same in men and as in women, after performing 10,000 simulations to correct for different degrees of association between each confounder and death, Shi et al. concluded that confounding is unlikely to account for the increased risk for mortality in women. Driven by the fact that observational studies suggest that females have poorer postoperative outcomes compared to males following cardiac surgery, Dixon et al.4 performed a systematic review and meta-analysis reporting short-term mortality (in-hospital/30 day), long-term mortality, postoperative stroke, sternal wound infection, or myocardial infarction in both sexes, after adjusting for baseline characteristics, following either CABG, heart valve surgery, or the two surgeries combined. The long-term mortality was found to be equivalent in both sexes, for all types of cardiac surgery. Females were found to be at a greater risk of short-term mortality and postoperative stroke than males following CABG or valve surgery combined with CABG, but not after isolated valve surgery. Finally, only a limited number of reviews summarize the published evidence regarding the risk difference between sexes of postoperative outcomes4,5.
The purpose of our meta-analysis was to evaluate whether differences between men and women could be better established in postoperative short-term outcomes after CABG, based on the evidence published in the past 5 years. For this, we analysed mortality after CABG, and also short-term outcomes, including the occurrence of stroke, myocardial infarction, and postoperative atrial fibrillation, which are all associated with significant morbidity and mortality6–9.
Results
We included 23 studies, with a total of 3,971,267 patients (70.7% men, 29.3% women) (Table 1). Twenty-two of these studies analysed existent institutional, national, or multinational clinical registries and administrative databases. Being retrospective in nature, these studies covered evidence collected over a wide time span, some starting as early as 1998. One study analysed pooled individual patient data from four randomized controlled trials10, and one study performed a subgroup analysis of TiCAB randomized controlled trial11. Among the selected studies, 15 used the variable “sex” to differentiate between men and women, seven used the term “gender”, and one study used “sex” and “gender” interchangeably.
Table 1.
Description of studies included in the meta-analysis.
| Author, year | Number of participants | Recruitment period | Geographical region/database | Intervention/patient selection |
|---|---|---|---|---|
| Gurram12 | 773 | 2015–2016 | India | First time isolated CABG |
| (132 women, 641 men) | Single centre, Kochi, Kerala | |||
| Johnston13 | 61,147 | 2008–2016 | Canada | CABG, isolated and concomitant with valve surgery |
| (13,043 women, 48,104 men) | CorHealth Ontario, Administrative health registries for residents of Ontario | ≥ 40 years old | ||
| ter Woorst14 | 17,919 | 1998–2016 | Netherlands | Isolated CABG |
| (4016 women, 13,903 men) | Single centre (the Catharina Hospital, Eindhoven) | with or without the use of extracorporeal circulation | ||
| Vrancic15 | 2979 | 2000–2017 | Argentina | isolated CABG |
| (299 women, 2680 men) | Single centre (Instituto Cardiovascular De Buenos Aires, Buenos Aires) | BITA grafts | ||
| Filardo16 | 9203 | 2002–2010 | USA | Isolated CABG |
| (2518 women, 6685men) | Multicentre (Baylor University Medical Center (Dallas, Tex), The Heart Hospital at Baylor Plano (Plano, Tex), Emory University (Atlanta, Ga), or Washington University (St Louis, Mo) | |||
| Mahowald17 | 424,338 | 2003–2016 | USA | CABG for AMI |
| (212,228 women, 212,110 men) | Nationwide Inpatient Sample (NIS) | |||
| Mohamed18 | 2,537,767 | 2004–2015 | USA | First time CABG, isolated and with concomitant valve surgery |
| (708,459 women, 1,829,308 men) | National Inpatient Sample (NIS) | |||
| Eris19 | 141 | 2015–2020 | Turkey | CABG with coronary endarterectomy |
| (46 women, 95 men) | Single centre: Bursa Yuksek Ihtisas Education and Research Hospital, Bursa | |||
| Gaudino10 | 13,193 | 2004–2020 | International | CABG |
| (2714 women, 10,479 men) | Individual patient data pooled from RCT: SYNTAX, EXCEL, NOBLE, FREEDOM | SYNTAX : CABG for patients with three-vessel or left main coronary artery disease | ||
| EXCEL : CABG left main coronary artery disease of low or intermediate anatomical complexity | ||||
| NOBLE: left main coronary artery disease | ||||
| FREEDOM: CABG in diabetic patients with multivessel disease | ||||
| Kytö20 | 3214 | 2004–2014 | Finland | First time isolated CABG |
| (1607 women, 1607 men) | Care Register for Healthcare in Finland (CRHF) | adult with ACS | ||
| Matyal21 | 6250 | 2002–2020 | USA | Isolated CABG |
| (1339 women, 4911 men) | Society of Thoracic Surgeons (STS) database | |||
| Chang22 | 22,692 | 2000–2013 | Taiwan | Isolated CABG, CABG concomitant with valve surgery |
| (11,346women, 11,346 men) | Taiwan National Health Insurance Research Database (NHIRD) | |||
| Dixon23 | 147,476 | 2010–2018 | UK | Isolated CABG, elective or urgent |
| (26,157 women, 121,319 men) | National Adult Cardiac Surgery Audit (NACSA) data, obtained from the National Institute of Cardiovascular Outcomes Research (NICOR) | |||
| Gerfer24 | 92 | 2006–2017 | Germany | Isolated CABG |
| (19 women, 73 men) | Single centre: Hospital of Cologne, Cologne | patients with PCS receiving vaECMO | ||
| Moroni25 | 886 | 2002–2015 | International | CABG for ULMCA disease |
| (324 women, 562 men) | Multicentre, DELTA and DELTA 2 registries | |||
| Ter Woorst26 | 7826 | 1998–2015 | Netherlands | Isolated CABG |
| (3913 women, 3913 men) | Single centre (the Catharina Hospital, Eindhoven) | With or without the use of extracorporeal circulation | ||
| Qu27 | 20,045 | 2002–2019 | USA | Isolated elective CABG |
| (5367 women, 14,678 men) | The Society of Thoracic Surgeons (STS) | |||
| Ram28 | 1308 | 2000–2016 | Israel | First time elective isolated CABG for ACS |
| (263 women, 1045 men) | ACS Israeli Survey (ACSIS) | |||
| Wang29 | 13,616 | 2009–2019 | China | CABG, isolated and concomitant with valve surgery, |
| (6808 women, 6808 men) | Single centre: Fuwai Hospital, Beijing | TXA administration | ||
| Daoulah30 | 916 | 2015–2019 | Arab Gulf Countries | Isolated CABG for ULMCA disease |
| (132 women, 784 men) | Gulf-LM registry | |||
| Gaudino31 | 673,977 | 2016–2020* | USA | Primary isolated CABG |
| (160,194 women, 513,783 men) | The Society of Thoracic Surgeons (STS) | |||
| Sajja32 | 3650 | 1999–2018 | India | First time isolated CABG |
| (1825 women, 1825 men) | Star Hospitals, Hiderabad | |||
| Sandner11 | 1859 | 2012–2018 | International | Isolated CABG |
| (280 women, 1579 men) | subgroup analysis of TiCAB RCT | patients with stable coronary artery disease or acute coronary syndrome undergoing CABG for three-vessel disease and/or left main stenosis, or two-vessel disease with impaired left ventricular function |
*1,297,204 patients (317,716 women, 979,488 men), between 2011 and 2020, only the data for 2016–2020 were included in our analysis, due to potential overlap with Mohamed, 202018.
ACS, acute coronary syndrome; AMI, acute myocardial infarction; BITA, bilateral internal thoracic artery; PCS, postcardiotomy cardiogenic shock; RCT, randomized control trials; TXA, tranexamic acid; ULMCA, unprotected left main coronary artery; vaECMO, veno-arterial extracorporeal membrane oxygenation.
Postoperative mortality
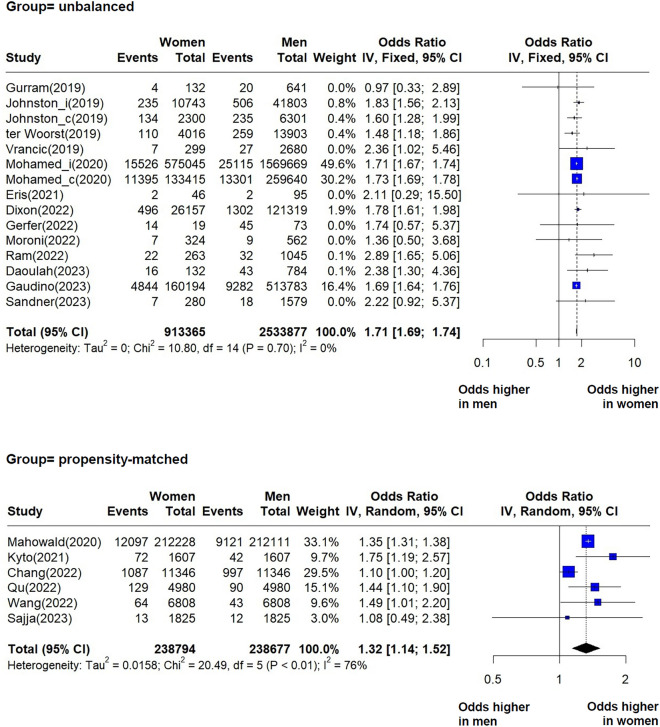
We identified and pooled data from 13 studies reporting unadjusted short-term mortality (in hospital or at 30 days) in unbalanced groups, and from six studies comparing propensity matched groups of women and men. Our findings indicate that women undergoing CABG are at higher risk of short-term mortality compared to men (Fig. 1). For the unbalanced group, OR = 1.71 (95% CI 1.69–1.74), with low heterogeneity between studies (I2 = 0%, p = 0.70). In contrast with the unbalanced groups, the propensity matched groups showed significant heterogeneity (Fig. 1) and a pooled effect OR = 1.32 (95% CI 1.14–1.52, I2 = 76%, p < 0.01). The mixed effects analysis between the two groups indicated a statistically significant effect difference (p < 0.01) (Supplementary material, Figs. S1–S3).
Fig. 1.
Forest plots showing pooled odds ratios (OR) for short-term mortality for each group, unbalanced and propensity-matched. When within individual studies outcomes were calculated separately by procedure: “_i” designates isolated CABG, and “_c” designates CABG concomitant with valve repair.
Postoperative stroke
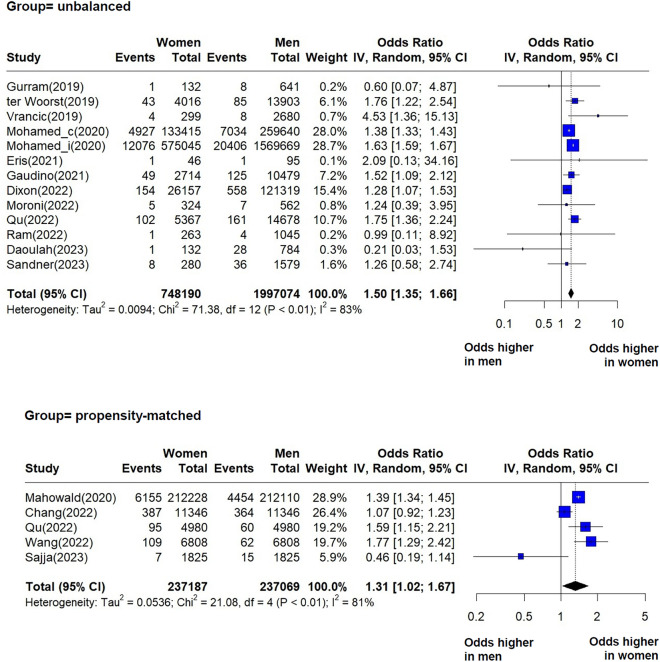
In the analysis of postoperative stroke, reported as either within hospital stroke or within 30 days from surgery, we included five studies that reported effects in propensity-matched groups of women and men, and 12 studies that reported unadjusted effects for postoperative stroke (both haemorrhagic or ischemic) after CABG (Fig. 2). Studies within both groups, propensity-matched and unbalanced, suffer from significant heterogeneity. The pooled effect estimates indicate in each case that women are at higher risk of stroke following CABG compared to men: OR = 1.50 (95% CI 1.35–1.66, I2 = 83%, p < 0.01), and OR = 1.31 (95% CI 1.02–1.67, I2 = 81%, p < 0.01). There was no statistically significant difference between the effects of the two groups (p = 0.32) (Supplementary material, Figs. S4–S6).
Fig. 2.
Forest plots showing pooled odds ratios (OR) for postoperative stroke (in hospital and 30-day results combined) following CABG, for each group, unbalanced and propensity-matched. When within-individual study outcomes were calculated separately by procedure, “_i” designates isolated CABG, and “_c” designates CABG concomitant with valve repair.
Postoperative myocardial infarction
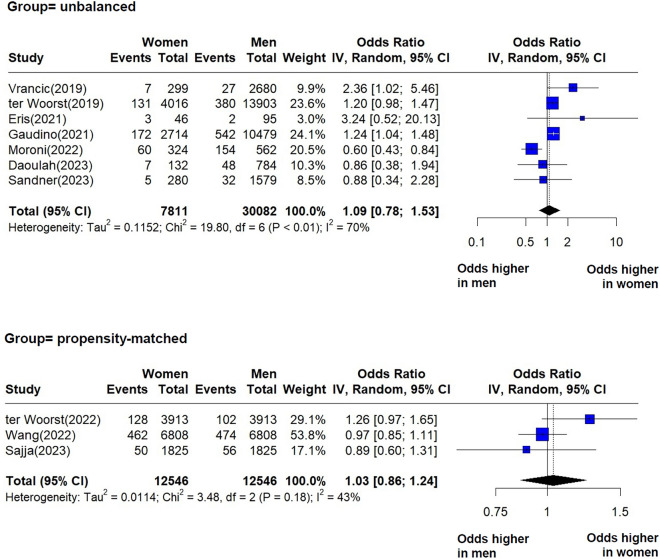
We identified postoperative myocardial infarction in seven studies of the unbalanced group and in three studies of the propensity matched group. We included both ST-elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI), as some studies did not specify the type of myocardial infarction. Our analysis indicated no statistically significant difference between women and men regarding the risk of myocardial infarction (Fig. 3). The pooled effects were OR = 1.09 (95% CI = 0.78–1.53, I2 = 70%, p < 0.01) and OR = 1.03 (95% CI = 0.86–1.24, I2 = 43%, p = 0.18), respectively. The I2 values indicate a moderate heterogeneity in each group. There was no statistically significant difference between the pooled effects of the two groups (p = 0.79) (Supplementary material, Figs. S7–S8).
Fig. 3.
Forest plots showing pooled odds ratios (OR) for postoperative myocardial infarction (in hospital and 30-day results combined) following CABG, for each group, unbalanced and propensity-matched.
Postoperative atrial fibrillation after CABG
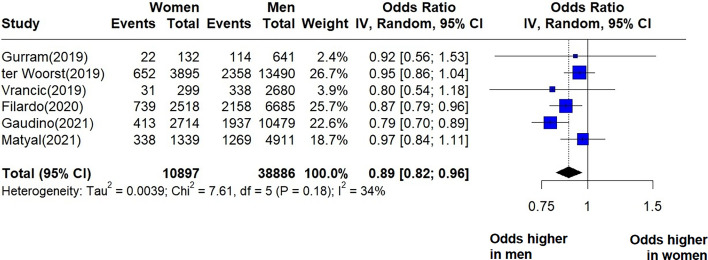
We identified six unbalanced studies reporting postoperative atrial fibrillation after CABG (Fig. 4). The pooled effect estimate indicates that women are at lower odds of postoperative atrial fibrillation: OR = 0.89 (95% CI = 0.82–0.96, I2 = 34%, p = 0.18). I2 values showed low-to-moderate heterogeneity between the studies. In four of these studies women were significantly older than men (Vrancic et al., ter Woorst et al., Filardo et al., Gaudino et al.), while in two studies there was no significant difference in age between the two groups (Gurram et al., Matyal et al.). Sensitivity analysis revealed that the source of heterogeneity came from the study of Gaudino et al., which contributed with a heavy weight to the summary of effect and had narrow uncertainty intervals (Supplementary material—Figs. S9, S10). We identified one propensity matched study (Sajja et al.) which indicated an OR = 0.97 (95% CI 0.73–1.27, p = 0.836) for postoperative atrial fibrillation in women compared to men.
Fig. 4.
Forest plot showing the pooled OR for postoperative atrial fibrillation in the short term (reported as in-hospital or 30-day outcome) following CABG.
Discussion
We identified discrepancies and similarities between women and men within the short-term period following CABG. Considering short-term mortality, our meta-analysis indicates that women are at higher risk of short-term mortality after CABG, and this finding is consistent with previously published reviews2–4. It has already been shown that short-term mortality risk is significantly and persistently higher in women, even after adjusting for age and co-morbidities2–4.
Sexual dimorphism influences aging-related alterations, leading to distinct cardiac and vascular phenotypes and outcomes. Disparities in myocardial and vascular biology between sexes have been documented, resulting in varied cardiovascular risk profiles throughout life. The diversity in sex specificities extends beyond genetic codes and expression, to encompass differences in stress response and aging trajectories33. In the general population, women are at higher risk of stroke, with more severe neurological deficits compared to men34,35. Our analysis confirms that after CABG, women are also at higher risk of stroke, whereas there is no significant difference in risk of myocardial infarction between men and women. The aetiology of stroke following CABG is diverse36, as is the aetiology of myocardial infarction—acute graft failure, technical factors related to graft manipulation, and concomitant valve surgery being the main contributors for the latter37. Although it is known that differences in anatomical features (smaller vessels, smaller volume of blood in women) explain why procedural factors and inflammatory responses to surgery are different in women compared to men, currently there is still no precise understanding of the degree to which these differences contribute to the different outcomes. Compared with the meta-analysis on the impact of sex on outcomes after cardiac surgery by Dixon et al.4, we chose to extract and summarize unadjusted effects of sex, and so we were able to include a larger number of, and more recent studies reporting on sex-specific post-operative outcomes for stroke and myocardial infarction. Interestingly, our findings regarding the post-operative stroke and myocardial infarction both in the propensity matched groups and in the unbalanced groups are similar to the findings of Dixon et al.4 in the analysis of studies reporting adjusted odds ratios.
We provide a summary of the unadjusted effect of postoperative atrial fibrillation, as reported in studies which analysed sex-specific characteristics and outcomes. Postoperative atrial fibrillation denotes new-onset atrial fibrillation in the postoperative hospitalization period38. Our results indicate that men are at higher risk of postoperative atrial fibrillation after CABG, even though women undergoing CABG are generally older and carry more co-morbidities. These results are consistent with previous studies. In their adjusted analysis, Filardo et al.16 found that the incidence of postoperative atrial fibrillation after CABG was higher in men compared to women, and also men had longer duration of their first atrial fibrillation episodes. Gaudino et al.10 also indicated that men after CABG were at higher risk of postoperative atrial fibrillation compared to women, with an adjusted odds ratio (men as reference) = 0.82 (95% CI 0.73–0.94). Conversely, neither the adjusted analysis by Matyal et al.21 nor the propensity matched study by Sajja et al.32 reported any statistically significant difference regarding postoperative atrial fibrillation between men and women.
Postoperative atrial fibrillation is an important outcome to be addressed in the postoperative setting. Atrial fibrillation is associated with graft failure39. Although our analysis indicates that women are not particularly at elevated risk of postoperative atrial fibrillation compared to men, this occurrence is still of concern because atrial fibrillation carries a higher risk of stroke in women compared to men34,40,41. Numerous studies elaborate predictive models for postoperative atrial fibrillation42–47 and analyse sex as co-variates. When reports do not consider the predictors for postoperative atrial fibrillation as being sex specific, the value of the findings can be offset. For example, the size of the left atrium (LA) is known to differ subtly between men and women48. The LA size is often identified as an independent predictor of postoperative atrial fibrillation after CABG49–53. Not analysing sex-dependent thresholds in LA size might hinder the validity and applicability of the findings49–52. In contrast, when sex-specific thresholds in the postoperative enlargement of LA are taken into account, they can be valuable for risk stratification53.
Given the complex differences that delineate the female patient population from the male patient population in the context of CABG, it is necessary to develop studies in which the baseline and intra-operative characteristics are analysed separately, in a sex-specific and age-dependent manner. The post-operative outcomes could be associated with sex different thresholds. For example, Khalagy et al.54 indicated that the levels of preoperative haemoglobin associated with in hospital mortality after CABG are different in men compared to women. Developing more studies that provide a differentiated analysis for male and female patients is crucial. This approach will increase the chance of obtaining accurate predictive models separately for men and women, meaningful in clinical practice and particularly in secondary cardiovascular prevention.
Limitations of our study stem from the following: first, we included studies which combine outcomes for isolated CABG and CABG concomitant with valve surgery. Second, it was not possible to perform a separate analysis for elective and urgent CABG, nor for procedural factors (such as on pump and off pump CABG, number of grafts, and others). Third, the studies with a propensity matched design use different criteria for matching the cohorts of men with the cohorts of women. In addition, for short-term mortality and postoperative stroke, the propensity-matched group and the unbalanced studies might have had overlapping databases. Fourth, we could not make the difference between haemorrhagic or ischemic post operative stroke as in some studies that was not specified. Fifth, the criteria used to define myocardial infarction differed among the studies, which could have contributed to the observed heterogeneity, and we could not differentiate between STEMI or NSTEMI. Sixth, in several studies, there is no mentioned definition for postoperative atrial fibrillation in terms of duration or assessment method. Also, in most of the studies we selected for analysis, it was not possible to obtain information regarding the incidence of preoperative atrial fibrillation in men, compared to women.
Conclusion
In our meta-analysis, the summaries of effects from unbalanced groups and propensity matched groups indicate that the gap between women and men regarding the short-term postoperative mortality risk persists, and that women are at higher risk of postoperative stroke. The available evidence does not indicate that the likelihood of postoperative myocardial infarction is different in women compared to men. Despite women undergoing CABG being generally older and having more co-morbidities compared to men, men remain at higher risk of postoperative atrial fibrillation.
Methods
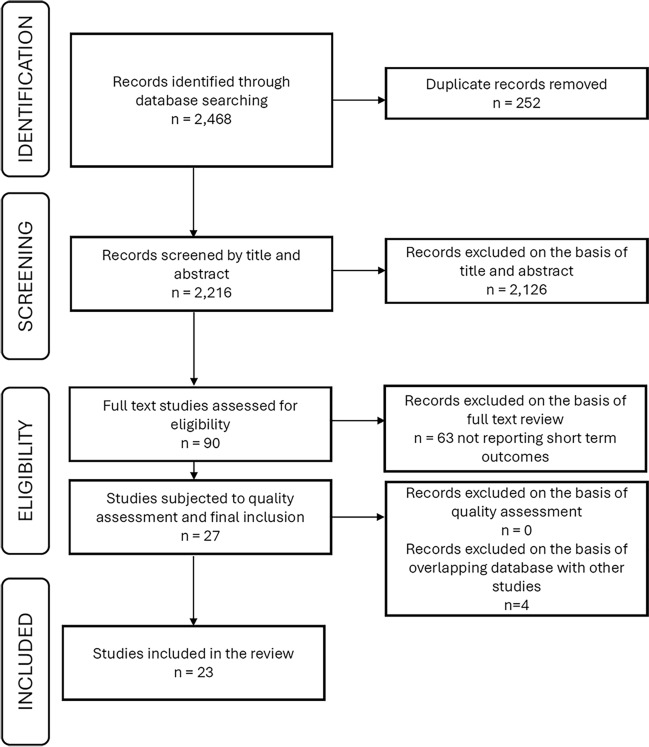
This meta-analysis was based on data extracted from previously published research and the data and study materials are available in the public domain. We performed a systematic literature search in PubMed database from January 1, 2019, to November 14, 2023. We used a combination of search terms (CABG, sex, gender, men, women, male, female, differences) and Boolean operators (“OR” for combining search terms for similar concepts, in parentheses, and “AND” to combine different concepts). Titles and abstracts were screened against the inclusion and exclusion criteria. Studies were included in the meta-analysis if they were observational studies or randomized clinical trials; published in English; presenting data regarding the following short-term outcomes after CABG: 30-day mortality, in hospital mortality, postoperative stroke (cardiovascular accidents), postoperative myocardial infarction (perioperative, early, in-hospital, and 30-day myocardial infarction), postoperative atrial fibrillation; reporting characteristics (baseline, intraoperative) and outcomes independently for males and females, following isolated CABG or CABG concomitant with other type of surgery; reporting effects unadjusted for baseline characteristics, or outcomes for propensity matched groups. Studies were excluded if they were protocols, reviews, case series, or referring to minimally invasive CABG. When source databases overlapped between the studies reporting the same category of outcomes, we selected and included in the statistical analysis only the study with the largest sample size. The flow chart depicting the study selection process is shown in Fig. 5.
Fig. 5.
Flow chart describing the study selection process.
The study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for abstracting data and assessing data quality and validity55. Two independent reviewers (DDL and ET) selected studies by screening titles, abstracts, and full texts of articles identified in this search. For eligible studies, data extraction was performed by two reviewers (DL and PR). The quality of the studies was assessed with the Newcastle-Ottawa Scale56 (Supplementary file, Table S1).
The extracted data were baseline characteristics (being a man or woman, age at the time of CABG), procedural characteristics (elective or urgent CABG), and short-term outcomes: in hospital mortality, 30-day mortality, and 30-day or in hospital stroke, myocardial infarction, and postoperative atrial fibrillation. Criteria used in each selected study for defining myocardial infarction are presented in Supplementary file, Table S2. Except for one study (Filardo et al.16), no explicit criteria were used for defining postoperative atrial fibrillation, Table S3. After extracting the number of events from the individual studies, we computed the pooled odds ratios (OR) for women vs. men, with 95% confidence intervals (CI), using the generic inverse variance method. Group analysis was performed by study design type (unbalanced groups, propensity matched groups of women compared to men). For investigating variance between the groups, for each outcome, we used the mixed effects model, consisting in analysis within the group using the random effect model, and analysis between the groups using the fixed effects model. Heterogeneity was considered low if I2 < 25%, and significant if I2 > 75%. For groups with low heterogeneity, we performed a separate analysis using the fixed effect model. When studies overlapped in terms of source database, we included in the group only the study with a larger sample size (narrower uncertainty interval). Statistical significance was considered for p < 0.05. We used the R ’meta’ and ‘dmetar’ packages (R version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria)57 and ‘metabin’ function58. To assess the robustness of our study, we conducted sensitivity analysis by leaving out studies with too broad or too narrow selection criteria, or outliers (Supplementary file). Funnel plots and Egger’s test were used to identify the publication bias (Supplementary file—Fig. S11).
Supplementary Information
Author contributions
D.D.L.: conceptualization of the study, screening and selection of the studies, data extraction, data analysis, wrote the original draft, reviewing and editing the manuscript. E.L.: screening and selection of the studies, contributions to the original draft, reviewing and editing the manuscript. P.R.: data extraction, reviewing and editing the manuscript. F.M.: reviewing and editing the manuscript. C.H.: conceptualization of the study, reviewing and editing the manuscript, acquired funding.
Funding
The study was supported by Valentine Gerbex-Bourget Foundation.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71414-2.
References
- 1.Melly, L., Torregrossa, G., Lee, T., Jansens, J. L. & Puskas, J. D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis.10(3), 1960–1967. 10.21037/jtd.2018.02.43 (2018). 10.21037/jtd.2018.02.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryce Robinson, N. et al. Sex differences in outcomes following coronary artery bypass grafting: A meta-analysis. Interact. Cardiovasc. Thorac. Surg.33(6), 841–847. 10.1093/icvts/ivab191 (2021). 10.1093/icvts/ivab191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi, D. et al. Higher mortality in women after coronary artery bypass: Meta-analysis and bias analysis of confounding. Ann. Thorac. Surg.113(2), 674–680. 10.1016/j.athoracsur.2020.11.039 (2022). 10.1016/j.athoracsur.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 4.Dixon, L. K. et al. Impact of sex on outcomes after cardiac surgery: A systematic review and meta-analysis. Int. J. Cardiol.343, 27–34. 10.1016/j.ijcard.2021.09.011 (2021). 10.1016/j.ijcard.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 5.Harik, L. et al. Sex differences in coronary artery bypass graft surgery outcomes: A narrative review. J. Thorac. Dis.15(9), 5041–5054. 10.21037/jtd-23-294 (2023). 10.21037/jtd-23-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner, B. D. et al. Factors associated with long-term survival in patients with stroke after coronary artery bypass grafting. J. Int. Med. Res.48(7), 300060520920428. 10.1177/0300060520920428 (2020). 10.1177/0300060520920428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litwinowicz, R. et al. Long-term survival following postoperative myocardial infraction after coronary artery bypass surgery. J. Thorac. Dis.14(1), 102–112. 10.21037/jtd-21-1279 (2022). 10.21037/jtd-21-1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin, M. H. et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality: A meta-analysis. Stroke50(6), 1364–1371. 10.1161/STROKEAHA.118.023921 (2019). 10.1161/STROKEAHA.118.023921 [DOI] [PubMed] [Google Scholar]
- 9.Alex Chau, Y. L. et al. The impact of post-operative atrial fibrillation on outcomes in coronary artery bypass graft and combined procedures. J. Geriatr. Cardiol. JGC18(5), 319–326. 10.11909/j.issn.1671-5411.2021.05.005 (2021). 10.11909/j.issn.1671-5411.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudino, M. et al. Sex differences in outcomes after coronary artery bypass grafting: A pooled analysis of individual patient data. Eur. Heart J.43(1), 18–28. 10.1093/eurheartj/ehab504 (2021). 10.1093/eurheartj/ehab504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandner, S. et al. Sex differences among patients receiving ticagrelor monotherapy or aspirin after coronary bypass surgery: A prespecified subgroup analysis of the TiCAB trial. Int. J. Cardiol.370, 129–135. 10.1016/j.ijcard.2022.10.166 (2023). 10.1016/j.ijcard.2022.10.166 [DOI] [PubMed] [Google Scholar]
- 12.Gurram, A. et al. Female gender is not a risk factor for early mortality after coronary artery bypass grafting. Ann. Card. Anaesth.22(2), 187–193. 10.4103/aca.ACA_27_18 (2019). 10.4103/aca.ACA_27_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston, A., Mesana, T. G., Lee, D. S., Eddeen, A. B. & Sun, L. Y. Sex differences in long-term survival after major cardiac surgery: A population-based cohort study. J. Am. Heart Assoc.8(17), e013260. 10.1161/JAHA.119.013260 (2019). 10.1161/JAHA.119.013260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ter Woorst, J. F., van Straten, A. H. M., Houterman, S. & Soliman-Hamad, M. A. Sex difference in coronary artery bypass grafting: Preoperative profile and early outcome. J. Cardiothorac. Vasc. Anesth.33(10), 2679–2684. 10.1053/j.jvca.2019.02.040 (2019). 10.1053/j.jvca.2019.02.040 [DOI] [PubMed] [Google Scholar]
- 15.Vrancic, J. M. et al. Is sex a risk factor for death in patients with bilateral internal thoracic artery grafts?. J. Thorac. Cardiovasc. Surg.158(5), 1345-1353.e1. 10.1016/j.jtcvs.2019.01.025 (2019). 10.1016/j.jtcvs.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 16.Filardo, G. et al. Postoperative atrial fibrillation: Sex-specific characteristics and effect on survival. J. Thorac. Cardiovasc. Surg.159(4), 1419-1425.e1. 10.1016/j.jtcvs.2019.04.097 (2020). 10.1016/j.jtcvs.2019.04.097 [DOI] [PubMed] [Google Scholar]
- 17.Mahowald, M. K., Alqahtani, F. & Alkhouli, M. Comparison of outcomes of coronary revascularization for acute myocardial infarction in men versus women. Am. J. Cardiol.132, 1–7. 10.1016/j.amjcard.2020.07.014 (2020). 10.1016/j.amjcard.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 18.Mohamed, W. et al. Trends in sex-based differences in outcomes following coronary artery bypass grafting in the United States between 2004 and 2015. Int. J. Cardiol.320, 42–48. 10.1016/j.ijcard.2020.07.039 (2020). 10.1016/j.ijcard.2020.07.039 [DOI] [PubMed] [Google Scholar]
- 19.Eris, C., Engin, M., Sunbul, S. A., As, A. K. & Erdolu, B. Early postoperative results of on-pump coronary endarterectomy: Is gender a risk factor?. Heart Surg. Forum24(4), E662–E669. 10.1532/hsf.3929 (2021). 10.1532/hsf.3929 [DOI] [PubMed] [Google Scholar]
- 20.Kytö, V., Sipilä, J., Rautava, P. & Gunn, J. Sex differences in outcomes following acute coronary syndrome treated with coronary artery bypass surgery. Heart Lung Circ.30(1), 100–107. 10.1016/j.hlc.2020.02.009 (2021). 10.1016/j.hlc.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 21.Matyal, R. et al. Update: Gender differences in CABG outcomes—Have we bridged the gap?. PLoS ONE16(9), e0255170. 10.1371/journal.pone.0255170 (2021). 10.1371/journal.pone.0255170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang, F. C. et al. Sex differences in risks of in-hospital and late outcomes after cardiac surgery: A nationwide population-based cohort study. BMJ Open12(2), e058538. 10.1136/bmjopen-2021-058538 (2022). 10.1136/bmjopen-2021-058538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon, L. K. et al. Females have an increased risk of short-term mortality after cardiac surgery compared to males: Insights from a national database. J. Card. Surg.37(11), 3507–3519. 10.1111/jocs.16928 (2022). 10.1111/jocs.16928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerfer, S. et al. Gender-related propensity score match analysis of ECMO therapy in postcardiotomy cardiogenic shock in patients after myocardial revascularization. Perfusion37(5), 470–476. 10.1177/02676591211004363 (2022). 10.1177/02676591211004363 [DOI] [PubMed] [Google Scholar]
- 25.Moroni, F. et al. Sex differences in outcomes after percutaneous coronary intervention or coronary artery bypass graft for left main disease: From the DELTA Registries. J. Am. Heart Assoc.11(5), e022320. 10.1161/JAHA.121.022320 (2022). 10.1161/JAHA.121.022320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ter Woorst, J. F., Olsthoorn, J. R., Houterman, S., van Straten, B. H. M. & Soliman-Hamad, M. A. Sex difference in long-term survival after coronary artery bypass grafting is age-dependent. J. Cardiothorac. Vasc. Anesth.36(5), 1288–1295. 10.1053/j.jvca.2021.08.104 (2022). 10.1053/j.jvca.2021.08.104 [DOI] [PubMed] [Google Scholar]
- 27.Qu, W. W., Wei, J. W., Binongo, J. N. & Keeling, W. B. Sex differences in failure-to-rescue after coronary artery bypass grafting. Ann. Thorac. Surg.114(5), 1596–1602. 10.1016/j.athoracsur.2021.09.070 (2022). 10.1016/j.athoracsur.2021.09.070 [DOI] [PubMed] [Google Scholar]
- 28.Ram, E. et al. Coronary artery bypass grafting following acute coronary syndrome: Impact of gender. Semin. Thorac. Cardiovasc. Surg.34(3), 920–929. 10.1053/j.semtcvs.2021.07.015 (2022). 10.1053/j.semtcvs.2021.07.015 [DOI] [PubMed] [Google Scholar]
- 29.Wang, E., Wang, Y., Hu, S. & Yuan, S. Impact of gender differences on hemostasis in patients after coronary artery bypass grafts surgeries in the context of tranexamic acid administration. J. Cardiothorac. Surg.17(1), 123. 10.1186/s13019-022-01874-y (2022). 10.1186/s13019-022-01874-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daoulah, A. et al. Does gender affect the outcomes of myocardial revascularization for left-main coronary artery disease?. Angiology75(2), 182–189. 10.1177/00033197231162481 (2023). 10.1177/00033197231162481 [DOI] [PubMed] [Google Scholar]
- 31.Gaudino, M. et al. Operative outcomes of women undergoing coronary artery bypass surgery in the US, 2011 to 2020. JAMA Surg.158(5), 494–502. 10.1001/jamasurg.2022.8156 (2023). 10.1001/jamasurg.2022.8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajja, L. R., Mannam, G., Kamtam, D. N. & Balakrishna, N. Female gender does not have any significant impact on the early postoperative outcomes after coronary artery bypass grafting: A propensity-matched analysis. Indian J. Thorac. Cardiovasc. Surg.39(3), 231–237. 10.1007/s12055-022-01465-5 (2023). 10.1007/s12055-022-01465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji, H. et al. Sex differences in myocardial and vascular aging. Circ. Res.130(4), 566–577. 10.1161/CIRCRESAHA.121.319902 (2022). 10.1161/CIRCRESAHA.121.319902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang, C. et al. Do women with atrial fibrillation experience more severe strokes?: Results from the Austrian Stroke Unit Registry. Stroke48(3), 778–780. 10.1161/STROKEAHA.116.015900 (2017). 10.1161/STROKEAHA.116.015900 [DOI] [PubMed] [Google Scholar]
- 35.Yoon, C. W. & Bushnell, C. D. Stroke in women: A review focused on epidemiology, risk factors, and outcomes. J. Stroke25(1), 2–15. 10.5853/jos.2022.03468 (2023). 10.5853/jos.2022.03468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaudino, M. et al. Stroke after coronary artery bypass grafting and percutaneous coronary intervention: Incidence, pathogenesis, and outcomes. J. Am. Heart Assoc.8(13), e013032. 10.1161/JAHA.119.013032 (2019). 10.1161/JAHA.119.013032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaudino, M. et al. Considerations on the management of acute postoperative ischemia after cardiac surgery: A scientific statement from the American Heart Association. Circulation148(5), 442–454. 10.1161/CIR.0000000000001154 (2023). 10.1161/CIR.0000000000001154 [DOI] [PubMed] [Google Scholar]
- 38.Perezgrovas-Olaria, R. et al. Differences in postoperative atrial fibrillation incidence and outcomes after cardiac surgery according to assessment method and definition: A systematic review and meta-analysis. J. Am. Heart Assoc.12(19), e030907. 10.1161/JAHA.123.030907 (2023). 10.1161/JAHA.123.030907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han, Z., Zhang, G. & Chen, Y. Early asymptomatic graft failure in coronary artery bypass grafting: A study based on computed tomography angiography analysis. J. Cardiothorac. Surg.18(1), 98. 10.1186/s13019-023-02199-0 (2023). 10.1186/s13019-023-02199-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emdin, C. A. et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. BMJ532, h7013. 10.1136/bmj.h7013 (2016). 10.1136/bmj.h7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cove, C. L. et al. Female sex as an independent risk factor for stroke in atrial fibrillation: Possible mechanisms. Thromb. Haemost.111(3), 385–391. 10.1160/TH13-04-0347 (2014). 10.1160/TH13-04-0347 [DOI] [PubMed] [Google Scholar]
- 42.Chen, Y. C. et al. Systemic immune-inflammation index for predicting postoperative atrial fibrillation following cardiac surgery: A meta-analysis. Front. Cardiovasc. Med.11, 1290610. 10.3389/fcvm.2024.1290610 (2024). 10.3389/fcvm.2024.1290610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mekonen Gdey, M. et al. Predictors of developing postoperative atrial fibrillation in patients undergoing coronary artery bypass graft: A systematic review and meta-analysis. Cureus15(12), e51316. 10.7759/cureus.51316 (2023). 10.7759/cureus.51316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo, E. J., Hong, J., Lee, H. J. & Son, Y. J. Perioperative risk factors for new-onset postoperative atrial fibrillation after coronary artery bypass grafting: A systematic review. BMC Cardiovasc. Disord.21(1), 418. 10.1186/s12872-021-02224-x (2021). 10.1186/s12872-021-02224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, R. J. Z. et al. A prediction model for new-onset atrial fibrillation following coronary artery bypass graft surgery: A multicenter retrospective study. Heliyon9(3), e14656. 10.1016/j.heliyon.2023.e14656 (2023). 10.1016/j.heliyon.2023.e14656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, Y. et al. Metabolic signatures in pericardial fluid and serum are associated with new-onset atrial fibrillation after isolated coronary artery bypass grafting. Transl. Res.256, 30–40. 10.1016/j.trsl.2023.01.001 (2023). 10.1016/j.trsl.2023.01.001 [DOI] [PubMed] [Google Scholar]
- 47.Serafim, K. R. et al. The accuracy of the Stroke Risk Analysis (SRA) system for predicting atrial fibrillation in patients in the postoperative period of myocardial revascularization. PLoS ONE18(3), e0282565. 10.1371/journal.pone.0282565 (2023). 10.1371/journal.pone.0282565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zemrak, F. et al. Left atrial structure in relationship to age, sex, ethnicity, and cardiovascular risk factors: MESA (Multi-Ethnic Study of Atherosclerosis). Circ. Cardiovasc. Imaging10(2), e005379. 10.1161/CIRCIMAGING.116.005379 (2017). 10.1161/CIRCIMAGING.116.005379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan, K. et al. Predicting new-onset postoperative atrial fibrillation following isolated coronary artery bypass grafting: Development and validation of a novel nomogram. Int. J. Gen. Med.15, 937–948. 10.2147/IJGM.S346339 (2022). 10.2147/IJGM.S346339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubanenko, O., Rubanenko, A. & Davydkin, I. Comprehensive analysis of factors associated with new episode of postoperative atrial fibrillation after coronary artery bypass graft surgery. Life13(10), 2035. 10.3390/life13102035 (2023). 10.3390/life13102035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, H. et al. Development and validation of a diagnostic model based on left atrial diameter to predict postoperative atrial fibrillation after off-pump coronary artery bypass grafting. J. Thorac. Dis.15(7), 3708–3725. 10.21037/jtd-22-1706 (2023). 10.21037/jtd-22-1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, S., Che, H., Fan, Y. & Jiang, S. Prediction of new onset postoperative atrial fibrillation using a simple Nomogram. J. Cardiothorac. Surg.18(1), 139. 10.1186/s13019-023-02198-1 (2023). 10.1186/s13019-023-02198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karimi, A. et al. Left atrial size; a missing component in scoring systems for predicting atrial fibrillation following coronary artery bypass surgery. Acta Cardiol. Sin.36(5), 456. 10.6515/ACS.202009_36(5).20181023A (2020). 10.6515/ACS.202009_36(5).20181023A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khalaji, A. et al. Mortality across the spectrum of hemoglobin level in patients undergoing surgical coronary revascularization. Clin. Cardiol.46(5), 535–542. 10.1002/clc.24004 (2023). 10.1002/clc.24004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann. Intern. Med.151(4), 264. 10.7326/0003-4819-151-4-200908180-00135 (2009). 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 56.https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 57.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023). https://www.R-project.org/.
- 58.RStudio Team. RStudio: Integrated Development for R (RStudio, PBC, 2020). http://www.rstudio.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.