Abstract
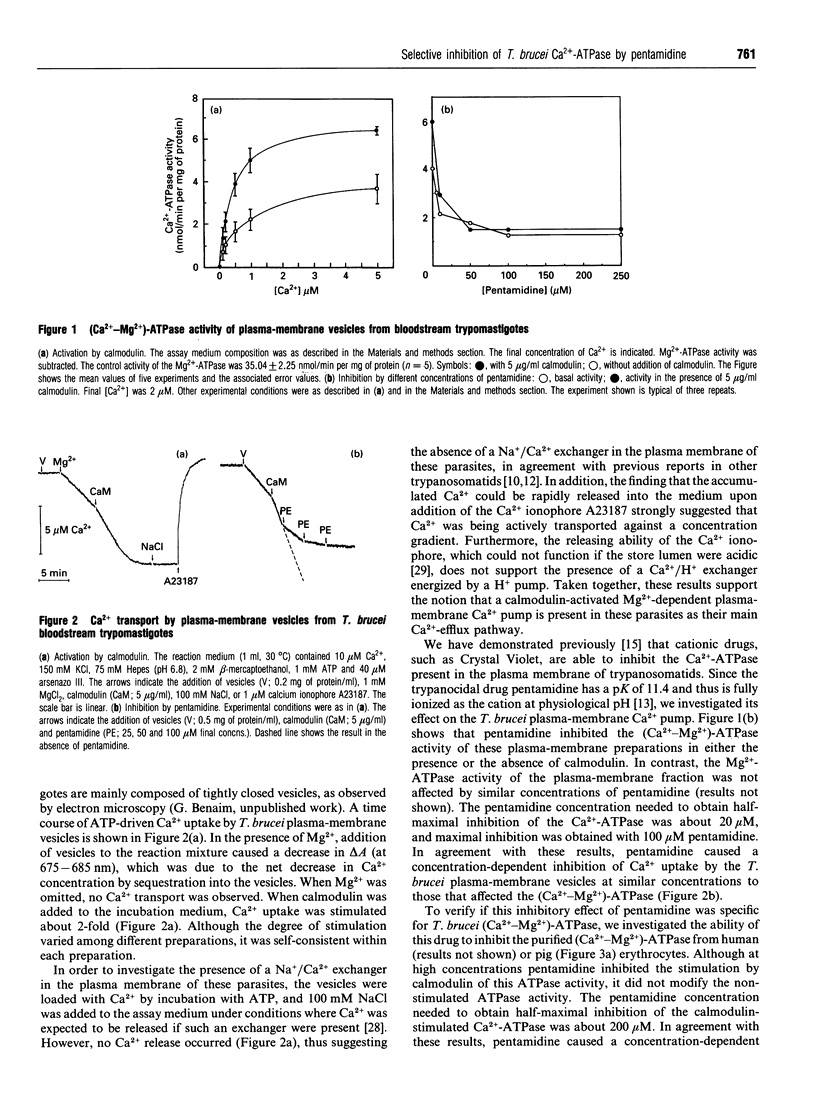
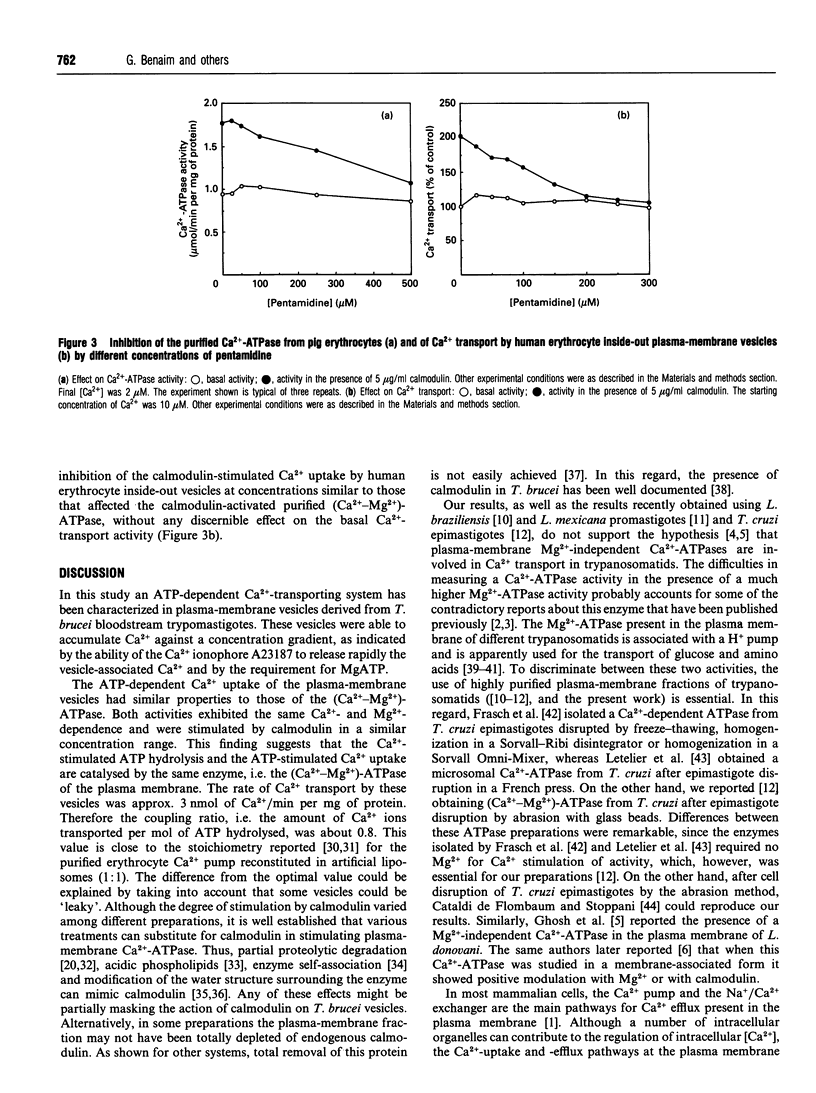
Despite previous reports [McLaughlin (1985) Mol. Biochem. Parasitol. 15, 189-201; Ghosh, Ray, Sarkar and Bhaduri (1990) J. Biol. Chem. 265, 11345-11351; Mazumder, Mukherjee, Ghosh, Ray and Bhaduri (1992) J. Biol. Chem. 267, 18440-18446] that the plasma membrane of different trypanosomatids only contains Ca(2+)-ATPase that does not show any demonstrable dependence on Mg2+, a high-affinity (Ca(2+)-Mg2+)-ATPase was demonstrated in the plasma membrane of Trypanosoma brucei. The enzyme became saturated with micromolar amounts of Ca2+, reaching a Vmax. of 3.45 +/- 0.66 nmol of ATP/min per mg of protein. The Km,app. for Ca2+ was 0.52 +/- 0.03 microM. This was decreased to 0.23 +/- 0.05 microM, and the Vmax. was increased to 6.36 +/- 0.22 nmol of ATP/min per mg of protein (about 85%), when calmodulin was present. T. brucei plasma-membrane vesicles accumulated Ca2+ on addition of ATP only when Mg2+ was present, and released it to addition of the Ca2+ ionophore A23187. In addition, this Ca2+ transport was stimulated by calmodulin. Addition of NaCl to Ca(2+)-loaded T. brucei plasma-membrane vesicles did not result in Ca2+ release, thus suggesting the absence of a Na+/Ca2+ exchanger in these parasites. Therefore the (Ca(2+)-Mg2+)-ATPase would be the only mechanism so far described that is responsible for the long-term fine tuning of the intracellular Ca2+ concentration of these parasites. The trypanocidal drug pentamidine inhibited the T. brucei plasma-membrane (Ca(2+)-Mg2+)-ATPase and Ca2+ transport at concentrations that had no effect on the Ca(2+)-ATPase activity of human or pig erythrocytes. In this latter case, pentamidine behaved as a weak calmodulin antagonist, since it inhibited the stimulation of the erythrocyte Ca(2+)-ATPase by calmodulin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benaim G., Cervino V., Hermoso T., Felibert P., Laurentin A. Intracellular calcium homeostasis in Leishmania mexicana. Identification and characterization of a plasma membrane calmodulin-dependent Ca(2+)-ATPase. Biol Res. 1993;26(1-2):141–150. [PubMed] [Google Scholar]
- Benaim G., Romero P. J. A calcium pump in plasma membrane vesicles from Leishmania braziliensis. Biochim Biophys Acta. 1990 Aug 10;1027(1):79–84. doi: 10.1016/0005-2736(90)90051-o. [DOI] [PubMed] [Google Scholar]
- Benaim G., Zurini M., Carafoli E. Different conformational states of the purified Ca2+-ATPase of the erythrocyte plasma membrane revealed by controlled trypsin proteolysis. J Biol Chem. 1984 Jul 10;259(13):8471–8477. [PubMed] [Google Scholar]
- Benaim G., de Meis L. Activation of the purified erythrocyte plasma membrane Ca2+- ATPase by organic solvents. FEBS Lett. 1989 Feb 27;244(2):484–486. doi: 10.1016/0014-5793(89)80589-7. [DOI] [PubMed] [Google Scholar]
- Benaim G., de Meis L. Similarities between the effects of dimethyl sulfoxide and calmodulin on the red blood cell Ca2(+)-ATPase. Biochim Biophys Acta. 1990 Jul 9;1026(1):87–92. doi: 10.1016/0005-2736(90)90336-m. [DOI] [PubMed] [Google Scholar]
- Bonay P., Cohen B. E. Neutral amino acid transport in Leishmania promastigotes. Biochim Biophys Acta. 1983 Jun 10;731(2):222–228. doi: 10.1016/0005-2736(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Zurini M. The Ca2+-pumping ATPase of plasma membranes. Purification, reconstitution and properties. Biochim Biophys Acta. 1982 Dec 31;683(3-4):279–301. doi: 10.1016/0304-4173(82)90004-0. [DOI] [PubMed] [Google Scholar]
- Cataldi de Flombaum M. A., Stoppani A. O. High-affinity calcium-stimulated, magnesium-dependent adenosine triphosphatase in Trypanosoma cruzi. Comp Biochem Physiol B. 1992 Dec;103(4):933–937. doi: 10.1016/0305-0491(92)90218-g. [DOI] [PubMed] [Google Scholar]
- Clark A., Carafoli E. The stoichiometry of the Ca2+-pumping ATPase of erythrocytes. Cell Calcium. 1983 Apr;4(2):83–88. doi: 10.1016/0143-4160(83)90037-4. [DOI] [PubMed] [Google Scholar]
- Cohen B. E., Ramos H., Gamargo M., Urbina J. The water and ionic permeability induced by polyene antibiotics across plasma membrane vesicles from Leishmania sp. Biochim Biophys Acta. 1986 Aug 7;860(1):57–65. doi: 10.1016/0005-2736(86)90498-0. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Ca2+ transport in nerve fibers. Biochim Biophys Acta. 1988 Oct 11;947(3):549–569. doi: 10.1016/0304-4157(88)90007-x. [DOI] [PubMed] [Google Scholar]
- Docampo R., Gadelha F. R., Moreno S. N., Benaim G., Hoffmann M. E., Vercesi A. E. Disruption of Ca2+ homeostasis in Trypanosoma cruzi by crystal violet. J Eukaryot Microbiol. 1993 May-Jun;40(3):311–316. doi: 10.1111/j.1550-7408.1993.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Segura E. L., Cazzulo J. J., Stoppani A. O. Adenosine triphosphatase activities in Trypanosoma cruzi. Comp Biochem Physiol B. 1978;60(3):271–275. doi: 10.1016/0305-0491(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Ghosh J., Ray M., Sarkar S., Bhaduri A. A high affinity Ca2(+)-ATPase on the surface membrane of Leishmania donovani promastigote. J Biol Chem. 1990 Jul 5;265(19):11345–11351. [PubMed] [Google Scholar]
- Guerini D., Krebs J., Carafoli E. Stimulation of the purified erythrocyte Ca2+-ATPase by tryptic fragments of calmodulin. J Biol Chem. 1984 Dec 25;259(24):15172–15177. [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kosk-Kosicka D., Bzdega T., Wawrzynow A. Fluorescence energy transfer studies of purified erythrocyte Ca2+-ATPase. Ca2+-regulated activation by oligomerization. J Biol Chem. 1989 Nov 25;264(33):19495–19499. [PubMed] [Google Scholar]
- Letelier M. E., Alliende C., González J., Aldunate J., Repetto Y., Morello A. Phosphatase activity in Trypanosoma cruzi. Phosphate removal from ATP, phosphorylated proteins and other phosphate compounds. Comp Biochem Physiol B. 1986;85(2):375–380. doi: 10.1016/0305-0491(86)90015-5. [DOI] [PubMed] [Google Scholar]
- Mazumder S., Mukherjee T., Ghosh J., Ray M., Bhaduri A. Allosteric modulation of Leishmania donovani plasma membrane Ca(2+)-ATPase by endogenous calmodulin. J Biol Chem. 1992 Sep 15;267(26):18440–18446. [PubMed] [Google Scholar]
- McLaughlin J. A high affinity Ca2+-dependent ATPase in the surface membrane of the bloodstream stage of Trypanosoma rhodesiense. Mol Biochem Parasitol. 1985 May;15(2):189–201. doi: 10.1016/0166-6851(85)90119-7. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Villa A., Volpe P., Pozzan T. Cellular sites of IP3 action. Adv Second Messenger Phosphoprotein Res. 1992;26:187–208. [PubMed] [Google Scholar]
- Moreno S. N., Docampo R., Vercesi A. E. Calcium homeostasis in procyclic and bloodstream forms of Trypanosoma brucei. Lack of inositol 1,4,5-trisphosphate-sensitive Ca2+ release. J Biol Chem. 1992 Mar 25;267(9):6020–6026. [PubMed] [Google Scholar]
- Moreno S. N., Vercesi A. E., Pignataro O. P., Docampo R. Calcium homeostasis in Trypanosoma cruzi amastigotes: presence of inositol phosphates and lack of an inositol 1,4,5-trisphosphate-sensitive calcium pool. Mol Biochem Parasitol. 1992 Jun;52(2):251–261. doi: 10.1016/0166-6851(92)90057-q. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Oz H. S., Wittner M., Tanowitz H. B., Bilezikian J. P., Saxon M., Morris S. A. Trypanosoma cruzi: mechanisms of intracellular calcium homeostasis. Exp Parasitol. 1992 Jun;74(4):390–399. doi: 10.1016/0014-4894(92)90201-k. [DOI] [PubMed] [Google Scholar]
- Roufogalis B. D., Akyempon C. K., Al-Jobore A., Minocherhomjee A. M. Regulation of the Ca2+ pump of the erythrocyte membrane. Ann N Y Acad Sci. 1982;402:349–367. doi: 10.1111/j.1749-6632.1982.tb25754.x. [DOI] [PubMed] [Google Scholar]
- Ruben L., Egwuagu C., Patton C. L. African trypanosomes contain calmodulin which is distinct from host calmodulin. Biochim Biophys Acta. 1983 Jul 29;758(2):104–113. doi: 10.1016/0304-4165(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Ruben L., Hutchinson A., Moehlman J. Calcium homeostasis in Trypanosoma brucei. Identification of a pH-sensitive non-mitochondrial calcium pool. J Biol Chem. 1991 Dec 25;266(36):24351–24358. [PubMed] [Google Scholar]
- Sands M., Kron M. A., Brown R. B. Pentamidine: a review. Rev Infect Dis. 1985 Sep-Oct;7(5):625–634. doi: 10.1093/clinids/7.5.625. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. Characteristics and regulation of active calcium transport in inside-out red cell membrane vesicles. Biochim Biophys Acta. 1980 May 23;598(2):326–338. doi: 10.1016/0005-2736(80)90010-3. [DOI] [PubMed] [Google Scholar]
- Scarpa A. Measurements of cation transport with metallochromic indicators. Methods Enzymol. 1979;56:301–338. doi: 10.1016/0076-6879(79)56030-3. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Renard D. C., Rooney T. A. Spatial organization of Ca2+ signalling and Ins(1,4,5)P3 action. Adv Second Messenger Phosphoprotein Res. 1992;26:225–263. [PubMed] [Google Scholar]
- Urbina J. A., Vivas J., Ramos H., Larralde G., Aguilar Z., Avilán L. Alteration of lipid order profile and permeability of plasma membranes from Trypanosoma cruzi epimastigotes grown in the presence of ketoconazole. Mol Biochem Parasitol. 1988 Aug;30(2):185–195. doi: 10.1016/0166-6851(88)90111-9. [DOI] [PubMed] [Google Scholar]
- Vercesi A. E., Docampo R. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem J. 1992 Jun 1;284(Pt 2):463–467. doi: 10.1042/bj2840463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi A. E., Moreno S. N., Bernardes C. F., Meinicke A. R., Fernandes E. C., Docampo R. Thapsigargin causes Ca2+ release and collapse of the membrane potential of Trypanosoma brucei mitochondria in situ and of isolated rat liver mitochondria. J Biol Chem. 1993 Apr 25;268(12):8564–8568. [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L. The Ca(2+)-transport ATPases from the plasma membrane. J Bioenerg Biomembr. 1992 Jun;24(3):285–300. doi: 10.1007/BF00768849. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Identification of a surface membrane proton-translocating ATPase in promastigotes of the parasitic protozoan Leishmania donovani. Biochem J. 1988 Nov 15;256(1):13–21. doi: 10.1042/bj2560013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Protonmotive force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]


