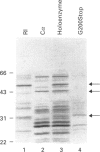
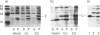Abstract
The type-I regulatory subunit (RI) of the cyclic AMP-dependent protein kinase (PKA) from Chinese hamster ovary (CHO) cells has been cloned and expressed in a strain of BL21(DE3) Escherichia coli lacking adenylate cyclase [BL21(DE3)/delta cya]. RI expressed in this bacterial system free of cyclic AMP is soluble and can reconstitute functional PKA. Recombinant CHO C alpha is predominantly insoluble with some active soluble protein. C beta is entirely insoluble and inactive. Soluble recombinant RI and soluble recombinant C alpha can associate in vitro and be activated by cyclic AMP. Six site-directed mutations of RI were generated to study the interaction of cyclic AMP with RI and RI-C alpha subunit interactions. Four cyclic AMP-binding-site point mutants were generated [W261R (tryptophan to arginine at position 261), a novel mutation in site A; V376G, a novel mutation in site B; G200E (site A), and Y370F (site B), previously described in bovine RI were introduced into the CHO RI for comparison purposes]. Mutants W261R, Y370F, and G200E demonstrated decreased 8-N3-[3H]cyclic AMP binding as well as 5-fold reduced affinity for [3H]cyclic AMP, with threefold increased EC50 values for cyclic AMP activation of kinase activity from reconstituted mutant holoenzymes. The mutation at V376G did not alter cyclic AMP binding or activation by cyclic AMP of mutant holoenzyme. A truncation mutant, G200Stop, which lacks both cyclic AMP-binding sites, did not bind cyclic AMP but can inhibit C alpha subunit activity. A novel mutation outside the cyclic AMP-binding regions of RI (V89A) weakened the interaction with C alpha indicated by a 7-fold lower EC50 for mutant holoenzyme activation by cyclic AMP.
Full text
PDF



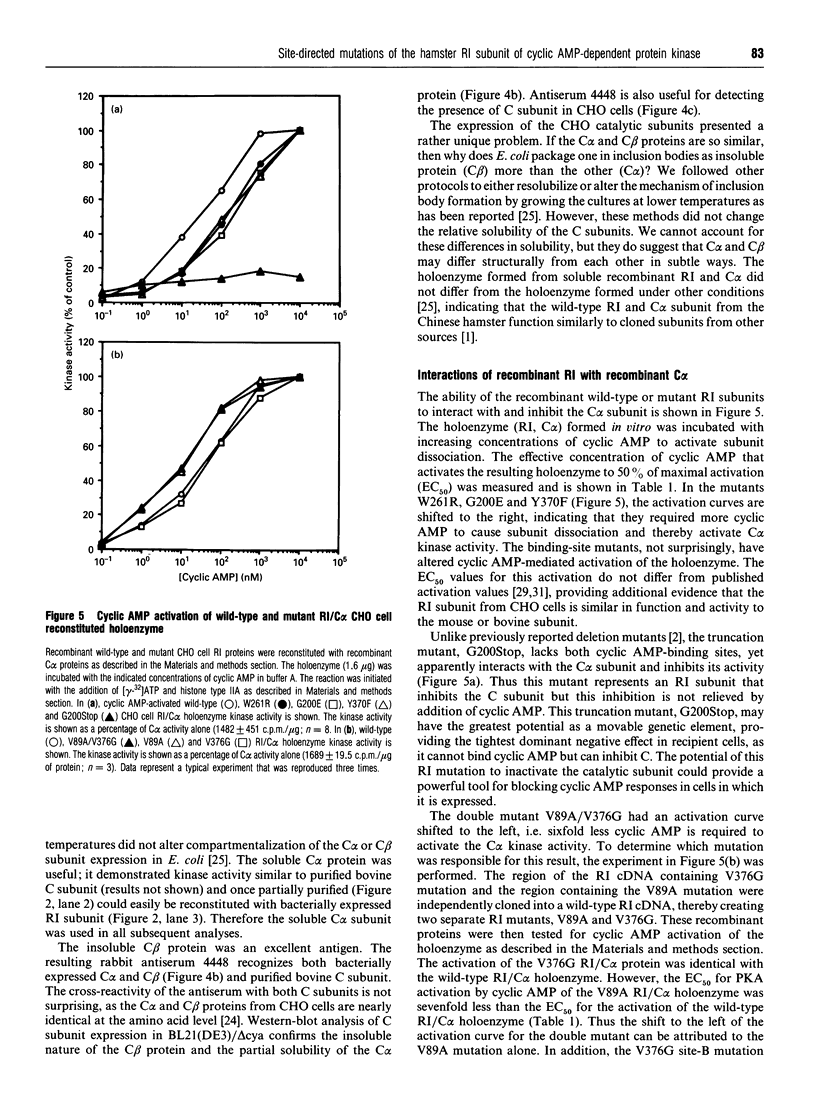
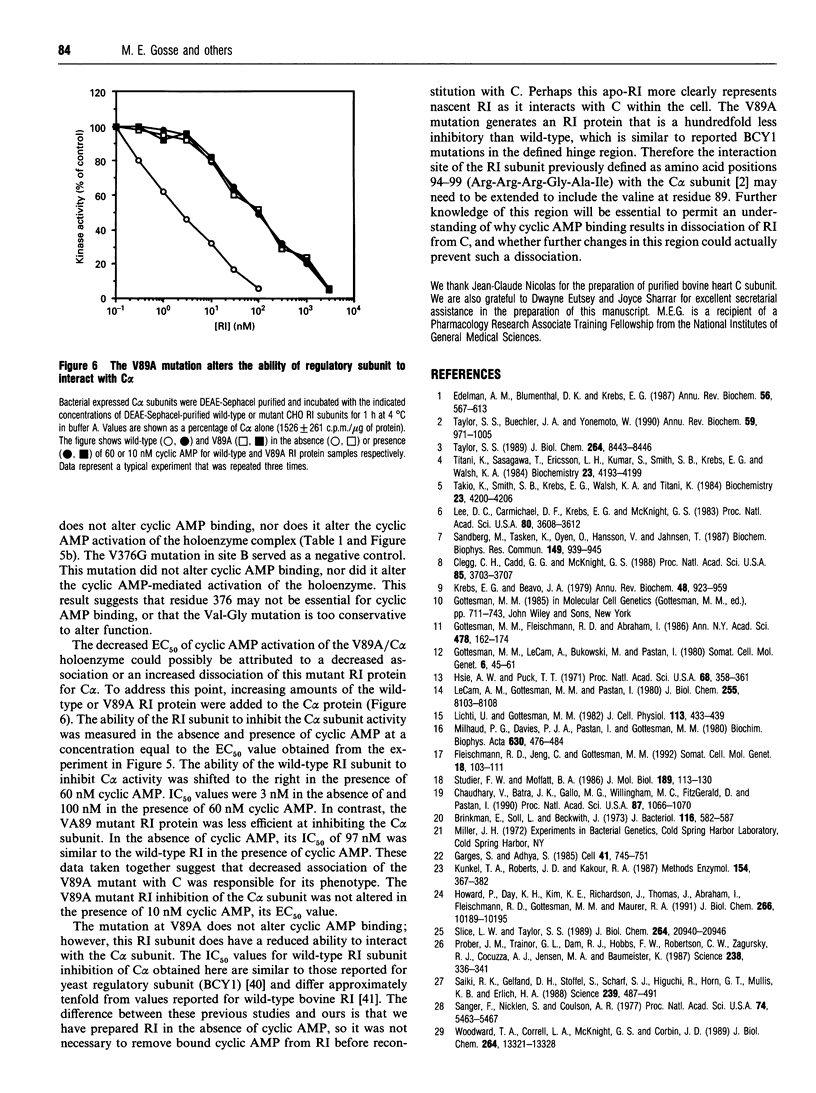

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann E. P., Chaudhary V., Gottesman M. M., Pastan I. Pseudomonas exotoxin fusion proteins are potent immunogens for raising antibodies against P-glycoprotein. Biotechniques. 1991 Feb;10(2):202-4, 206, 208-9. [PubMed] [Google Scholar]
- Bubis J., Saraswat L. D., Taylor S. S. Tyrosine-371 contributes to the positive cooperativity between the two cAMP binding sites in the regulatory subunit of cAMP-dependent protein kinase I. Biochemistry. 1988 Mar 8;27(5):1570–1576. doi: 10.1021/bi00405a026. [DOI] [PubMed] [Google Scholar]
- Buechler Y. J., Taylor S. S. Mutations in the autoinhibitor site of the regulatory subunit of cAMP-dependent protein kinase I. Replacement of Ala-97 and Ser-99 interferes with reassociation with the catalytic subunit. J Biol Chem. 1991 Feb 25;266(6):3491–3497. [PubMed] [Google Scholar]
- Chaudhary V. K., Batra J. K., Gallo M. G., Willingham M. C., FitzGerald D. J., Pastan I. A rapid method of cloning functional variable-region antibody genes in Escherichia coli as single-chain immunotoxins. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1066–1070. doi: 10.1073/pnas.87.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C. H., Cadd G. G., McKnight G. S. Genetic characterization of a brain-specific form of the type I regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3703–3707. doi: 10.1073/pnas.85.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C. H., Correll L. A., Cadd G. G., McKnight G. S. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987 Sep 25;262(27):13111–13119. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fleischmann R. D., Jeng C., Gottesman M. M. Ablation of stimulation of a cAMP-responsive promoter in CHO cell lines defective in their cAMP-dependent protein kinase system. Somat Cell Mol Genet. 1992 Mar;18(2):103–111. doi: 10.1007/BF01233157. [DOI] [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Fleischmann R., Abraham I. Molecular genetic analysis of cAMP-dependent protein kinase. Ann N Y Acad Sci. 1986;478:162–174. doi: 10.1111/j.1749-6632.1986.tb15529.x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., LeCam A., Bukowski M., Pastan I. Isolation of multiple classes of mutants of CHO cells resistant to cyclic AMP. Somatic Cell Genet. 1980 Jan;6(1):45–61. doi: 10.1007/BF01538695. [DOI] [PubMed] [Google Scholar]
- Howard P., Day K. H., Kim K. E., Richardson J., Thomas J., Abraham I., Fleischmann R. D., Gottesman M. M., Maurer R. A. Decreased catalytic subunit mRNA levels and altered catalytic subunit mRNA structure in a cAMP-resistant Chinese hamster ovary cell line. J Biol Chem. 1991 Jun 5;266(16):10189–10195. [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuret J., Johnson K. E., Nicolette C., Zoller M. J. Mutagenesis of the regulatory subunit of yeast cAMP-dependent protein kinase. Isolation of site-directed mutants with altered binding affinity for catalytic subunit. J Biol Chem. 1988 Jul 5;263(19):9149–9154. [PubMed] [Google Scholar]
- LeCam A., Gottesman M. M., Pastan I. Mechanism of cyclic AMP effect on nutrient transport in Chinese hamster ovary cells. A genetic approach. J Biol Chem. 1980 Sep 10;255(17):8103–8108. [PubMed] [Google Scholar]
- Lee D. C., Carmichael D. F., Krebs E. G., McKnight G. S. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3608–3612. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U., Gottesman M. M. Genetic evidence that a phorbol ester tumor promoter stimulates ornithine decarboxylase activity by a pathway that is independent of cyclic AMP-dependent protein kinases in CHO cells. J Cell Physiol. 1982 Dec;113(3):433–439. doi: 10.1002/jcp.1041130312. [DOI] [PubMed] [Google Scholar]
- Milhaud P. G., Davies P. J., Pastan I., Gottesman M. M. Regulation of transglutaminase activity in Chinese hamster ovary cells. Biochim Biophys Acta. 1980 Jul 15;630(4):476–484. doi: 10.1016/0304-4165(80)90002-1. [DOI] [PubMed] [Google Scholar]
- Ogreid D., Døskeland S. O., Gorman K. B., Steinberg R. A. Mutations that prevent cyclic nucleotide binding to binding sites A or B of type I cyclic AMP-dependent protein kinase. J Biol Chem. 1988 Nov 25;263(33):17397–17404. [PubMed] [Google Scholar]
- Prober J. M., Trainor G. L., Dam R. J., Hobbs F. W., Robertson C. W., Zagursky R. J., Cocuzza A. J., Jensen M. A., Baumeister K. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987 Oct 16;238(4825):336–341. doi: 10.1126/science.2443975. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic AMP-dependent protein kinase from bovine heart muscle. Methods Enzymol. 1974;38:308–315. doi: 10.1016/0076-6879(74)38047-0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Taskén K., Oyen O., Hansson V., Jahnsen T. Molecular cloning, cDNA structure and deduced amino acid sequence for a type I regulatory subunit of cAMP-dependent protein kinase from human testis. Biochem Biophys Res Commun. 1987 Dec 31;149(3):939–945. doi: 10.1016/0006-291x(87)90499-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. J., Hochman J., Verna R., Chapman M., Abraham I., Pastan I. H., Gottesman M. M. Characterization of a cyclic AMP-resistant Chinese hamster ovary cell mutant containing both wild-type and mutant species of type I regulatory subunit of cyclic AMP-dependent protein kinase. J Biol Chem. 1985 Nov 15;260(26):13927–13933. [PubMed] [Google Scholar]
- Slice L. W., Taylor S. S. Expression of the catalytic subunit of cAMP-dependent protein kinase in Escherichia coli. J Biol Chem. 1989 Dec 15;264(35):20940–20946. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Amino acid sequence of the regulatory subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4200–4206. doi: 10.1021/bi00313a029. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Taylor S. S. cAMP-dependent protein kinase. Model for an enzyme family. J Biol Chem. 1989 May 25;264(15):8443–8446. [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Ericsson L. H., Kumar S., Smith S. B., Krebs E. G., Walsh K. A. Amino acid sequence of the regulatory subunit of bovine type I adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4193–4199. doi: 10.1021/bi00313a028. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford T. A., Correll L. A., McKnight G. S., Corbin J. D. Expression and characterization of mutant forms of the type I regulatory subunit of cAMP-dependent protein kinase. The effect of defective cAMP binding on holoenzyme activation. J Biol Chem. 1989 Aug 5;264(22):13321–13328. [PubMed] [Google Scholar]