Abstract
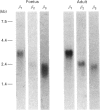
Possible modifications of the beta-adrenergic effector system during the development of bovine perirenal brown adipose tissue (BAT) in utero and its transformation into white-like adipose tissue after birth were studied. The parameters assessed were the level of expression of beta 1-, beta 2- and beta 3-adrenergic receptor (AR) mRNAs and the response of the plasma-membrane adenylate cyclase to (-)-isoprenaline and to the beta 3-agonist BRL 37344. The beta 3-AR mRNA was found to be expressed very early in utero, i.e. before the third month of foetal life. Then it increased dramatically (9-fold) between month 6 of foetal life and birth. A high beta 3-AR mRNA level was maintained after birth up to an age of 3 months. After conversion of BAT into white-like adipose tissue, i.e. in the adult bovine, the beta 3-AR mRNA expression became small or not detectable, and the beta 1-AR mRNA, which was expressed much less than the beta 3-AR mRNA in foetal life, became predominant. A response of the adenylate cyclase to (-)-isoprenaline was observed in foetal life (3.1-fold stimulation). It decreased after birth (1.8-fold stimulation) and then remained constant until adulthood. A response to BRL 37344 was also observed in foetal life (1.8-fold stimulation). It was maintained after birth, but disappeared in the adult. A possible relationship between the beta-AR expression and the adenylate cyclase response to (-)-isoprenaline on the one hand and the uncoupling-protein expression on the other is discussed. The bovine might represent a good model to understand the transition from brown to white fat in the human.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteilla L., Champigny O., Bouillaud F., Robelin J., Ricquier D. Sequential changes in the expression of mitochondrial protein mRNA during the development of brown adipose tissue in bovine and ovine species. Sudden occurrence of uncoupling protein mRNA during embryogenesis and its disappearance after birth. Biochem J. 1989 Feb 1;257(3):665–671. doi: 10.1042/bj2570665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteilla L., Forest C., Robelin J., Ricquier D., Lombet A., Ailhaud G. Characterization of mitochondrial-uncoupling protein in bovine fetus and newborn calf. Am J Physiol. 1987 May;252(5 Pt 1):E627–E636. doi: 10.1152/ajpendo.1987.252.5.E627. [DOI] [PubMed] [Google Scholar]
- Champigny O., Holloway B. R., Ricquier D. Regulation of UCP gene expression in brown adipocytes differentiated in primary culture. Effects of a new beta-adrenoceptor agonist. Mol Cell Endocrinol. 1992 Jul;86(1-2):73–82. doi: 10.1016/0303-7207(92)90177-8. [DOI] [PubMed] [Google Scholar]
- Champigny O., Ricquier D., Blondel O., Mayers R. M., Briscoe M. G., Holloway B. R. Beta 3-adrenergic receptor stimulation restores message and expression of brown-fat mitochondrial uncoupling protein in adult dogs. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10774–10777. doi: 10.1073/pnas.88.23.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F. Z., Lentes K. U., Gocayne J., Fitzgerald M., Robinson D., Kerlavage A. R., Fraser C. M., Venter J. C. Cloning and sequence analysis of the human brain beta-adrenergic receptor. Evolutionary relationship to rodent and avian beta-receptors and porcine muscarinic receptors. FEBS Lett. 1987 Jan 26;211(2):200–206. doi: 10.1016/0014-5793(87)81436-9. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Schlegel W., Lin M. C., Rodbell M. The fat cell adenylate cyclase system. Characterization and manipulation of its bimodal regulation by GTP. J Biol Chem. 1979 Sep 25;254(18):8927–8931. [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D., Pairault J. Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem. 1991 Oct 25;266(30):20329–20336. [PubMed] [Google Scholar]
- Giacobino J. P. Subcellular fractionation of brown adipose tissue. J Supramol Struct. 1979;11(4):445–449. doi: 10.1002/jss.400110403. [DOI] [PubMed] [Google Scholar]
- Giralt M., Casteilla L., Viñas O., Mampel T., Iglesias R., Robelin J., Villarroya F. Iodothyronine 5'-deiodinase activity as an early event of prenatal brown-fat differentiation in bovine development. Biochem J. 1989 Apr 15;259(2):555–559. doi: 10.1042/bj2590555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman J. G. Effects of agonist exposure on the coupling of beta 1 and beta 3 adrenergic receptors to adenylyl cyclase in isolated adipocytes. J Pharmacol Exp Ther. 1992 May;261(2):638–642. [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol. 1991 Dec;40(6):895–899. [PubMed] [Google Scholar]
- Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990 Aug;4(11):2890–2898. [PubMed] [Google Scholar]
- Huttunen P., Hirvonen J., Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46(4):339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- Klaus S., Cassard-Doulcier A. M., Ricquier D. Development of Phodopus sungorus brown preadipocytes in primary cell culture: effect of an atypical beta-adrenergic agonist, insulin, and triiodothyronine on differentiation, mitochondrial development, and expression of the uncoupling protein UCP. J Cell Biol. 1991 Dec;115(6):1783–1790. doi: 10.1083/jcb.115.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. H., Reviczky A., Chou P., Padbury J., Fisher D. A. Development of brown adipose tissue thermogenesis in the ovine fetus and newborn. Endocrinology. 1983 May;112(5):1662–1666. doi: 10.1210/endo-112-5-1662. [DOI] [PubMed] [Google Scholar]
- Krief S., Lönnqvist F., Raimbault S., Baude B., Van Spronsen A., Arner P., Strosberg A. D., Ricquier D., Emorine L. J. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993 Jan;91(1):344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langin D., Portillo M. P., Saulnier-Blache J. S., Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol. 1991 Jul 9;199(3):291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- Lean M. E., James W. P., Jennings G., Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes. 1986;10(3):219–227. [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J Biol Chem. 1988 Apr 15;263(11):4993–4996. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bunzow J. R., Searles R. P., Van Tol H., Tester B., Neve K. A., Teal P., Nipper V., Civelli O. Molecular cloning and expression of the rat beta 1-adrenergic receptor gene. J Biol Chem. 1990 Aug 5;265(22):12960–12965. [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Fraser C. M., Giacobino J. P. Radioligand binding studies of the atypical beta 3-adrenergic receptor in rat brown adipose tissue using [3H]CGP 12177. FEBS Lett. 1992 Feb 24;298(2-3):162–164. doi: 10.1016/0014-5793(92)80046-j. [DOI] [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem. 1991 Dec 15;266(35):24053–24058. [PubMed] [Google Scholar]
- Muzzin P., Seydoux J., Giacobino J. P., Venter J. C., Fraser C. Discrepancies between the affinities of binding and action of the novel beta-adrenergic agonist BRL 37344 in rat brown adipose tissue. Biochem Biophys Res Commun. 1988 Oct 14;156(1):375–382. doi: 10.1016/s0006-291x(88)80851-9. [DOI] [PubMed] [Google Scholar]
- Nahmias C., Blin N., Elalouf J. M., Mattei M. G., Strosberg A. D., Emorine L. J. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991 Dec;10(12):3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Padbury J. F., Diakomanolis E. S., Hobel C. J., Perelman A., Fisher D. A. Neonatal adaptation: sympatho-adrenal response to umbilical cord cutting. Pediatr Res. 1981 Dec;15(12):1483–1487. doi: 10.1203/00006450-198112000-00005. [DOI] [PubMed] [Google Scholar]
- Revelli J. P., Muzzin P., Paoloni A., Moinat M., Giacobino J. P. Expression of the beta 3-adrenergic receptor in human white adipose tissue. J Mol Endocrinol. 1993 Apr;10(2):193–197. doi: 10.1677/jme.0.0100193. [DOI] [PubMed] [Google Scholar]
- Ricquier D., Bouillaud F., Toumelin P., Mory G., Bazin R., Arch J., Pénicaud L. Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid beta-adrenoreceptor-mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem. 1986 Oct 25;261(30):13905–13910. [PubMed] [Google Scholar]
- Ricquier D., Casteilla L., Bouillaud F. Molecular studies of the uncoupling protein. FASEB J. 1991 Jun;5(9):2237–2242. doi: 10.1096/fasebj.5.9.1860614. [DOI] [PubMed] [Google Scholar]
- Ricquier D., Nechad M., Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab. 1982 Apr;54(4):803–807. doi: 10.1210/jcem-54-4-803. [DOI] [PubMed] [Google Scholar]
- Robelin J. Cellularity of bovine adipose tissues: developmental changes from 15 to 65 percent mature weight. J Lipid Res. 1981 Mar;22(3):452–457. [PubMed] [Google Scholar]
- Vatnick I., Tyzbir R. S., Welch J. G., Hooper A. P. Regression of brown adipose tissue mitochondrial function and structure in neonatal goats. Am J Physiol. 1987 Mar;252(3 Pt 1):E391–E395. doi: 10.1152/ajpendo.1987.252.3.E391. [DOI] [PubMed] [Google Scholar]