Abstract
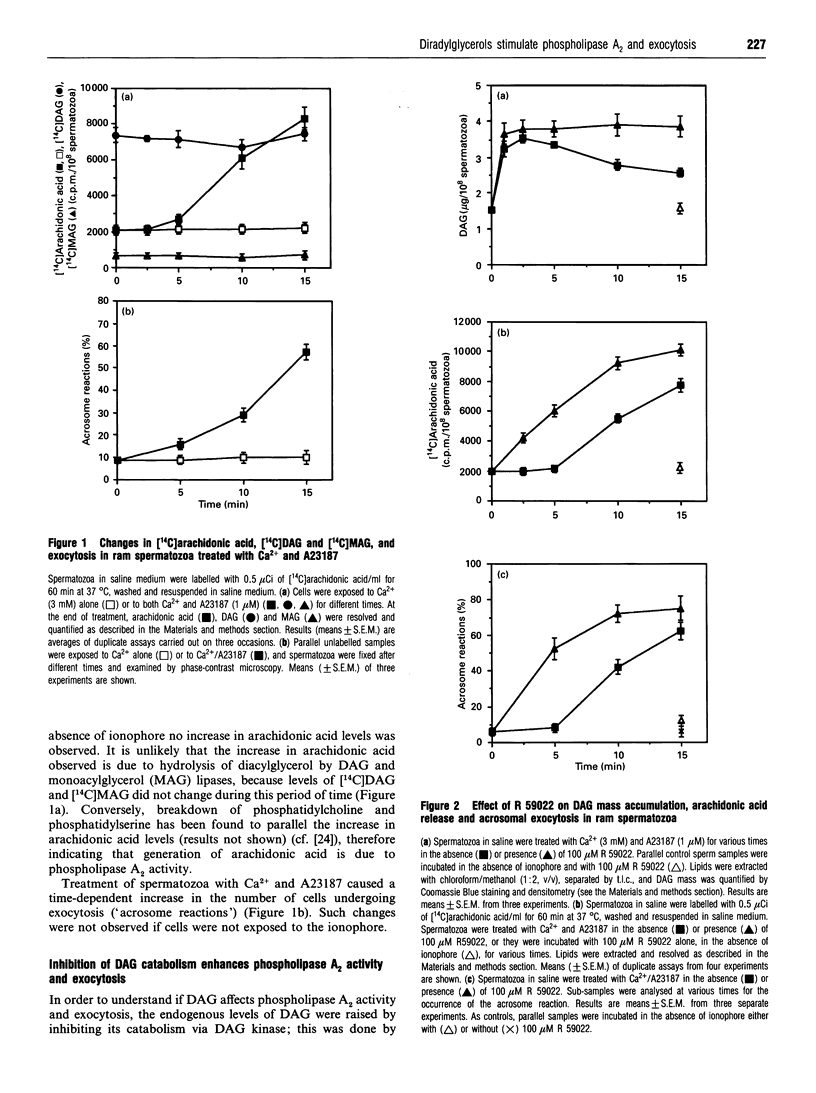
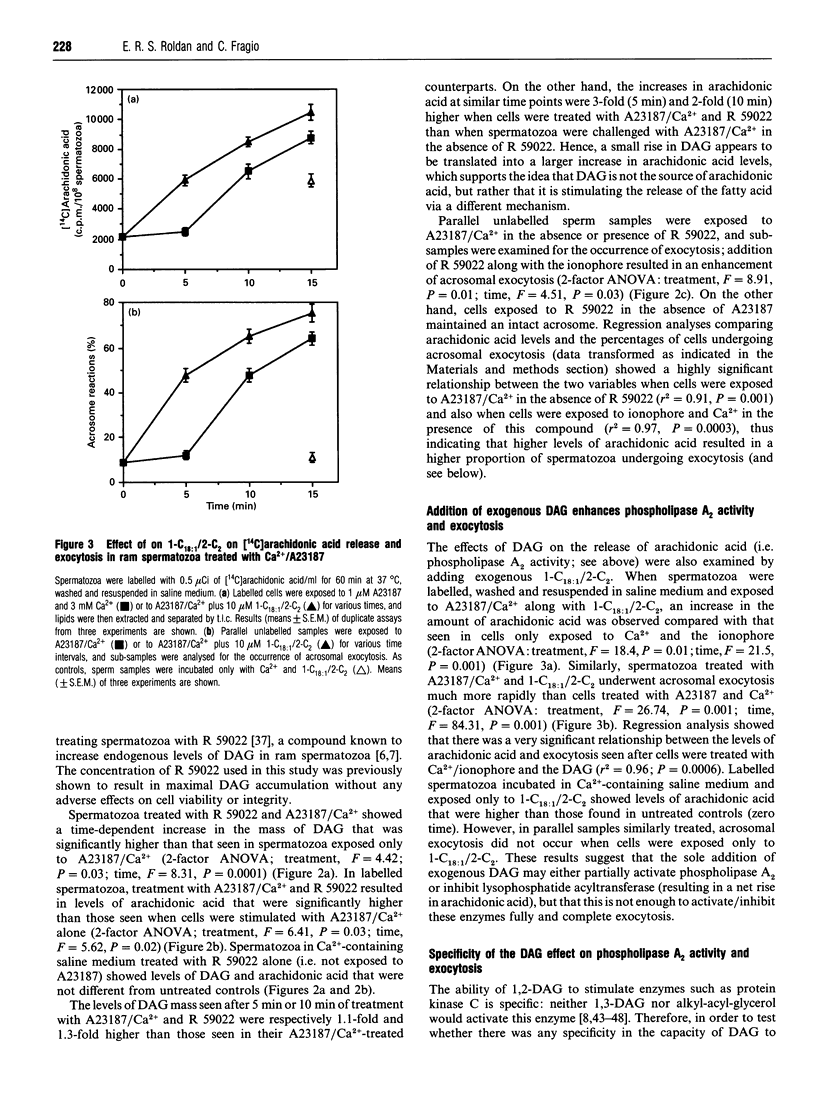
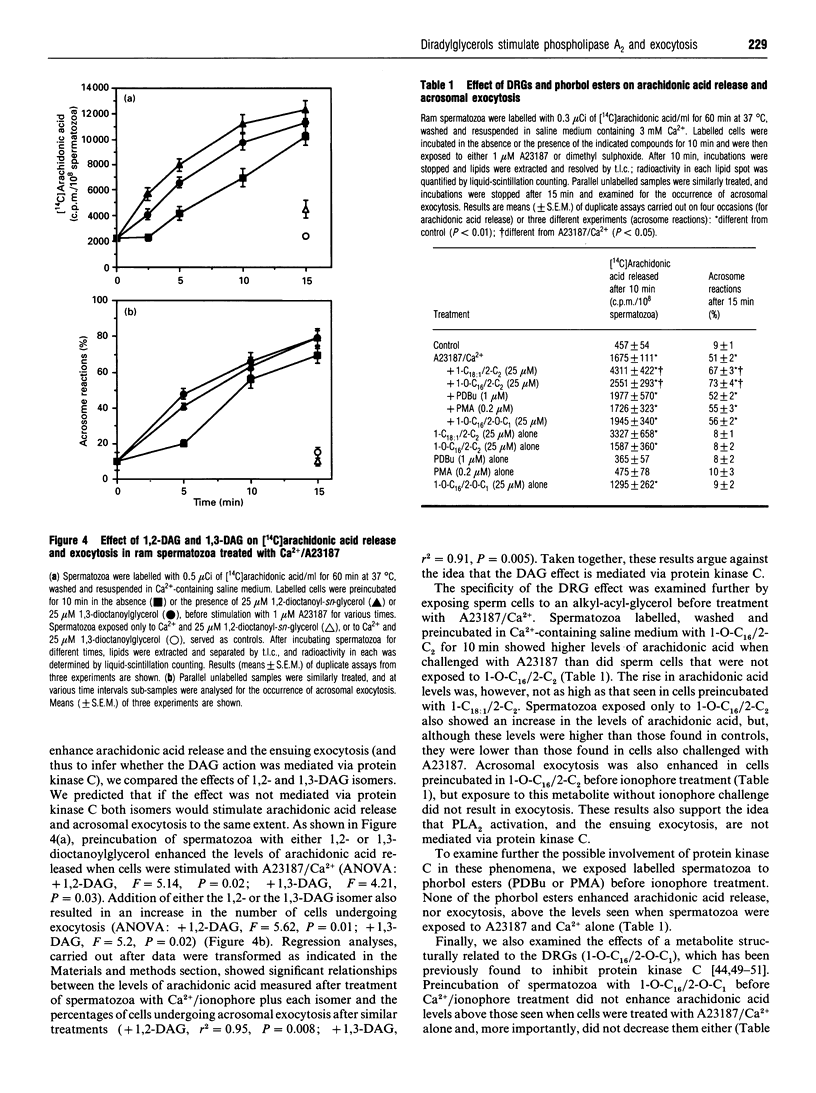
We tested the hypothesis that the role of diacylglycerol (DAG) in sperm acrosomal exocytosis is related to the activation of phospholipase A2, and that this effect is not mediated via protein kinase C. Treatment of [14C]arachidonic acid-labelled ram spermatozoa with Ca2+ and the ionophore A23187 stimulated both liberation of arachidonic acid and acrosomal exocytosis. No changes in [14C]DAG or [14C]monoacylglycerol were found after stimulation of spermatozoa, thus suggesting that arachidonic acid may be released exclusively via phospholipase A2. An increase in the endogenous levels of diradylglycerols (DRGs), resulting from exposure either to the DAG kinase inhibitor R 59022 or to exogenous 1-oleoyl-2-acetyl-sn-glycerol or 1,2-dioctanoyl-sn-glycerol, led to an increase in both phospholipase A2 activity and exocytosis when cells were stimulated with A23187 and Ca2+. Addition of DRGs that do not stimulate protein kinase C(1,3-dioctanoylglycerol, 1-O-hexadecyl-2-acetyl-rac-glycerol) also resulted in an increase in phospholipase A2 activity and exocytosis. On the other hand, phorbol esters (phorbol 12,13-dibutyrate; phorbol 12-myristate 13-acetate) did not enhance enzyme activity or exocytosis. Finally, exposure to 1-O-hexadecyl-2-O-methyl-rac-glycerol, a compound known to inhibit protein kinase C, did not affect phospholipase A2 activity or acrosomal exocytosis. We therefore conclude that in spermatozoa the messenger role of DAG is related to the activation of phospholipase A2, which in turn would generate an array of metabolites directly or indirectly involved in bringing about exocytosis of the acrosome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Gerard C., Olbrantz P., Wilson J., McCall C. E., McPhail L. C. Priming of the respiratory burst of neutrophils by diacylglycerol. Independence from activation or translocation of protein kinase C. J Biol Chem. 1987 May 15;262(14):6643–6649. [PubMed] [Google Scholar]
- Bauldry S. A., Wykle R. L., Bass D. A. Differential actions of diacyl- and alkylacylglycerols in priming phospholipase A2, 5-lipoxygenase and acetyltransferase activation in human neutrophils. Biochim Biophys Acta. 1991 Jul 9;1084(2):178–184. doi: 10.1016/0005-2760(91)90218-7. [DOI] [PubMed] [Google Scholar]
- Bauldry S. A., Wykle R. L., Bass D. A. Phospholipase A2 activation in human neutrophils. Differential actions of diacylglycerols and alkylacylglycerols in priming cells for stimulation by N-formyl-Met-Leu-Phe. J Biol Chem. 1988 Nov 15;263(32):16787–16795. [PubMed] [Google Scholar]
- Bennet P. J., Moatti J. P., Mansat A., Ribbes H., Cayrac J. C., Pontonnier F., Chap H., Douste-Blazy L. Evidence for the activation of phospholipases during acrosome reaction of human sperm elicited by calcium ionophore A23187. Biochim Biophys Acta. 1987 Jun 23;919(3):255–265. doi: 10.1016/0005-2760(87)90265-7. [DOI] [PubMed] [Google Scholar]
- Bhat B. G., Bardes E. S., Coleman R. A. Solubilization and partial purification of neonatally expressed rat hepatic microsomal monoacylglycerol acyltransferase. Arch Biochem Biophys. 1993 Feb 1;300(2):663–669. doi: 10.1006/abbi.1993.1092. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Siegel M. I. Phospholipase A2 activation in chemotactic peptide-stimulated HL60 granulocytes: synergism between diacylglycerol and Ca2+ in a protein kinase C-independent mechanism. Biochem Biophys Res Commun. 1987 Apr 29;144(2):683–691. doi: 10.1016/s0006-291x(87)80019-0. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Burch R. M. Diacylglycerol stimulates phospholipase A2 from Swiss 3T3 fibroblasts. FEBS Lett. 1988 Jul 18;234(2):283–286. doi: 10.1016/0014-5793(88)80099-1. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Jaken S. Structural and chemical specificity of diacylglycerols for protein kinase C activation. Biochem Biophys Res Commun. 1984 Nov 30;125(1):163–169. doi: 10.1016/s0006-291x(84)80349-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Nielson C. P., Stutchfield J. Is phospholipase A2 activation regulated by G-proteins? Biochem Soc Trans. 1991 Apr;19(2):333–336. doi: 10.1042/bst0190333. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Haynes E. B. Monoacylglycerol acyltransferase. Evidence that the activities from rat intestine and suckling liver are tissue-specific isoenzymes. J Biol Chem. 1986 Jan 5;261(1):224–228. [PubMed] [Google Scholar]
- Coleman R. A., Walsh J. P., Millington D. S., Maltby D. A. Stereospecificity of monoacylglycerol acyltransferase activity from rat intestine and suckling rat liver. J Lipid Res. 1986 Feb;27(2):158–165. [PubMed] [Google Scholar]
- Creutz C. E. The annexins and exocytosis. Science. 1992 Nov 6;258(5084):924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Small G. W., Schmitt J. D., Marasco C. J., Ishaq K., Piantadosi C. Alkyl-linked diglycerides inhibit protein kinase C activation by diacylglycerols. Biochem Biophys Res Commun. 1988 Feb 29;151(1):291–297. doi: 10.1016/0006-291x(88)90592-x. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Domino S. E., Garbers D. L. Stimulation of phospholipid turnover in isolated sea urchin sperm heads by the fucose-sulfate glycoconjugate that induces an acrosome reaction. Biol Reprod. 1989 Jul;41(1):133–141. doi: 10.1095/biolreprod41.1.133. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Wijkander J., Sundler R. Diacylglycerol induces deacylation of phosphatidylinositol and mobilization of arachidonic acid in mouse macrophages. Comparison with induction by phorbol diester. Biochem J. 1986 Nov 1;239(3):685–690. doi: 10.1042/bj2390685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. E., Hanley M. R. The role of phospholipases and phospholipid-derived signals in cell activation. Curr Opin Cell Biol. 1991 Apr;3(2):206–212. doi: 10.1016/0955-0674(91)90140-t. [DOI] [PubMed] [Google Scholar]
- Fleming A. D., Yanagimachi R. Evidence suggesting the importance of fatty acids and the fatty acid moieties of sperm membrane phospholipids in the acrosome reaction of guinea pig spermatozoa. J Exp Zool. 1984 Mar;229(3):485–489. doi: 10.1002/jez.1402290317. [DOI] [PubMed] [Google Scholar]
- Ford D. A., Miyake R., Glaser P. E., Gross R. W. Activation of protein kinase C by naturally occurring ether-linked diglycerides. J Biol Chem. 1989 Aug 15;264(23):13818–13824. [PubMed] [Google Scholar]
- Goppelt-Strübe M., Pfannkuche H. J., Gemsa D., Resch K. The diacylglycerols dioctanoylglycerol and oleoylacetylglycerol enhance prostaglandin synthesis by inhibition of the lysophosphatide acyltransferase. Biochem J. 1987 Nov 1;247(3):773–777. doi: 10.1042/bj2470773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A., Dott H. M., Foster G. C. Bovine serum albumin, sperm motility, and the "dilution effect'. J Exp Zool. 1982 Jul 20;222(1):81–88. doi: 10.1002/jez.1402220111. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Roldan E. R. Phosphoinositides and their products in the mammalian sperm acrosome reaction. J Reprod Fertil Suppl. 1990;42:51–67. [PubMed] [Google Scholar]
- Hata Y., Ogata E., Kojima I. Platelet-derived growth factor stimulates synthesis of 1,2-diacylglycerol from monoacylglycerol in Balb/c 3T3 cells. Biochem J. 1989 Sep 15;262(3):947–952. doi: 10.1042/bj2620947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkovska V. T., Momchilova A. B., Petkova D. H., Koumanov K. S. Phospholipase A2 activity in ram spermatozoa plasma membranes. Int J Biochem. 1987;19(6):569–572. doi: 10.1016/0020-711x(87)90143-1. [DOI] [PubMed] [Google Scholar]
- Imai A., Iida K., Matsunami K., Matsuda T., Tamaya T. Evidence for tight coupling of phospholipase activation and Ca2+ influx during acrosome reaction of golden hamster spermatozoa. Comp Biochem Physiol B. 1990;95(3):635–639. doi: 10.1016/0305-0491(90)90033-p. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Cao W. F. Properties of monoglycerol acyltransferase in rat adipocytes. Arch Biochem Biophys. 1992 Aug 1;296(2):419–425. doi: 10.1016/0003-9861(92)90592-k. [DOI] [PubMed] [Google Scholar]
- Kramer I. M., van der Bend R. L., Tool A. T., van Blitterswijk W. J., Roos D., Verhoeven A. J. 1-O-hexadecyl-2-Q-methylglycerol, a novel inhibitor of protein kinase C, inhibits the respiratory burst in human neutrophils. J Biol Chem. 1989 Apr 5;264(10):5876–5884. [PubMed] [Google Scholar]
- Kramer R. M., Checani G. C., Deykin D. Stimulation of Ca2+-activated human platelet phospholipase A2 by diacylglycerol. Biochem J. 1987 Dec 15;248(3):779–783. doi: 10.1042/bj2480779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröner E. E., Peskar B. A., Fischer H., Ferber E. Control of arachidonic acid accumulation in bone marrow-derived macrophages by acyltransferases. J Biol Chem. 1981 Apr 25;256(8):3690–3697. [PubMed] [Google Scholar]
- Langlais J., Chafouleas J. G., Ingraham R., Vigneault N., Roberts K. D. The phospholipase A2 of human spermatozoa; purification and partial sequence. Biochem Biophys Res Commun. 1992 Jan 15;182(1):208–214. doi: 10.1016/s0006-291x(05)80132-9. [DOI] [PubMed] [Google Scholar]
- Liscovitch M. Crosstalk among multiple signal-activated phospholipases. Trends Biochem Sci. 1992 Oct;17(10):393–399. doi: 10.1016/0968-0004(92)90007-v. [DOI] [PubMed] [Google Scholar]
- Llanos M. N., Lui C. W., Meizel S. Studies of phospholipase A2 related to the hamster sperm acrosome reaction. J Exp Zool. 1982 May 20;221(1):107–117. doi: 10.1002/jez.1402210114. [DOI] [PubMed] [Google Scholar]
- Meizel S., Turner K. O. Stimulation of an exocytotic event, the hamster sperm acrosome reaction, by cis-unsaturated fatty acids. FEBS Lett. 1983 Sep 19;161(2):315–318. doi: 10.1016/0014-5793(83)81032-1. [DOI] [PubMed] [Google Scholar]
- Molleyres L. P., Rando R. R. Structural studies on the diglyceride-mediated activation of protein kinase C. J Biol Chem. 1988 Oct 15;263(29):14832–14838. [PubMed] [Google Scholar]
- Morash S. C., Cook H. W., Spence M. W. Lysophosphatidylcholine as an intermediate in phosphatidylcholine metabolism and glycerophosphocholine synthesis in cultured cells: an evaluation of the roles of 1-acyl- and 2-acyl-lysophosphatidylcholine. Biochim Biophys Acta. 1989 Aug 8;1004(2):221–229. doi: 10.1016/0005-2760(89)90271-3. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Relationship between arachidonic acid release and Ca2(+)-dependent exocytosis in digitonin-permeabilized bovine adrenal chromaffin cells. Biochem J. 1990 Nov 1;271(3):571–574. doi: 10.1042/bj2710571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Handa S. Coomassie brilliant blue staining of lipids on thin-layer plates. Anal Biochem. 1984 Nov 1;142(2):406–410. doi: 10.1016/0003-2697(84)90484-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Howell J. I., Lucy J. A. Lysolecithin and cell fusion. Nature. 1970 Aug 22;227(5260):810–814. doi: 10.1038/227810a0. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Regulation of protein kinase C activity by lipids. FASEB J. 1988 May;2(8):2348–2355. doi: 10.1096/fasebj.2.8.3282960. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Absence of active protein kinase C in ram spermatozoa. Biochem Biophys Res Commun. 1988 Sep 15;155(2):901–906. doi: 10.1016/s0006-291x(88)80581-3. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Diacylglycerol and phosphatidate production and the exocytosis of the sperm acrosome. Biochem Biophys Res Commun. 1990 Oct 15;172(1):8–15. doi: 10.1016/s0006-291x(05)80165-2. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Polyphosphoinositide breakdown and subsequent exocytosis in the Ca2+/ionophore-induced acrosome reaction of mammalian spermatozoa. Biochem J. 1989 Apr 15;259(2):397–406. doi: 10.1042/bj2590397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. The role of diacylglycerol in the exocytosis of the sperm acrosome. Studies using diacylglycerol lipase and diacylglycerol kinase inhibitors and exogenous diacylglycerols. Biochem J. 1992 Feb 1;281(Pt 3):767–773. doi: 10.1042/bj2810767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan E. R., Mollinedo F. Diacylglycerol stimulates the Ca2(+)-dependent phospholipase A2 of ram spermatozoa. Biochem Biophys Res Commun. 1991 Apr 15;176(1):294–300. doi: 10.1016/0006-291x(91)90923-u. [DOI] [PubMed] [Google Scholar]
- Schonhardt T., Ferber E. Translocation of phospholipase A2 from cytosol to membranes induced by 1-oleoyl-2-acetyl-glycerol in serum-free cultured macrophages. Biochem Biophys Res Commun. 1987 Dec 16;149(2):769–775. doi: 10.1016/0006-291x(87)90434-7. [DOI] [PubMed] [Google Scholar]
- Simpson C. M., Itabe H., Reynolds C. N., King W. C., Glomset J. A. Swiss 3T3 cells preferentially incorporate sn-2-arachidonoyl monoacylglycerol into sn-1-stearoyl-2-arachidonoyl phosphatidylinositol. J Biol Chem. 1991 Aug 25;266(24):15902–15909. [PubMed] [Google Scholar]
- Thakkar J. K., East J., Franson R. C. Modulation of phospholipase A2 activity associated with human sperm membranes by divalent cations and calcium antagonists. Biol Reprod. 1984 Apr;30(3):679–686. doi: 10.1095/biolreprod30.3.679. [DOI] [PubMed] [Google Scholar]
- Thakkar J. K., East J., Seyler D., Franson R. C. Surface-active phospholipase A2 in mouse spermatozoa. Biochim Biophys Acta. 1983 Nov 1;754(1):44–50. doi: 10.1016/0005-2760(83)90080-2. [DOI] [PubMed] [Google Scholar]
- Thomas P., Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J. 1989 Dec 1;264(2):539–546. doi: 10.1042/bj2640539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Ryeom S. W., Picard S. T., Ackerman S. J., Dvorak A. M. Cytoplasmic lipid bodies of neutrophils: formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J Cell Biol. 1991 Apr;113(1):137–146. doi: 10.1083/jcb.113.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]
- van Blitterswijk W. J., van der Bend R. L., Kramer I. M., Verhoeven A. J., Hilkmann H., de Widt J. A metabolite of an antineoplastic ether phospholipid may inhibit transmembrane signalling via protein kinase C. Lipids. 1987 Nov;22(11):842–846. doi: 10.1007/BF02535541. [DOI] [PubMed] [Google Scholar]


