Abstract
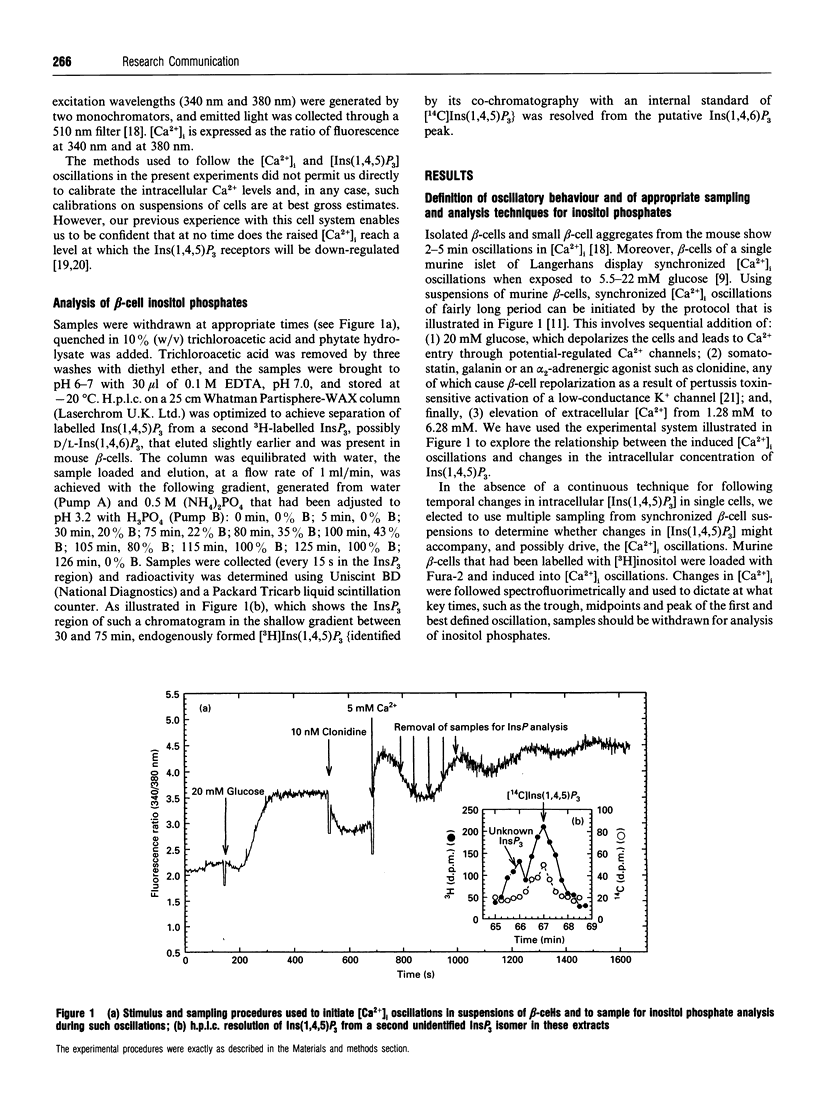
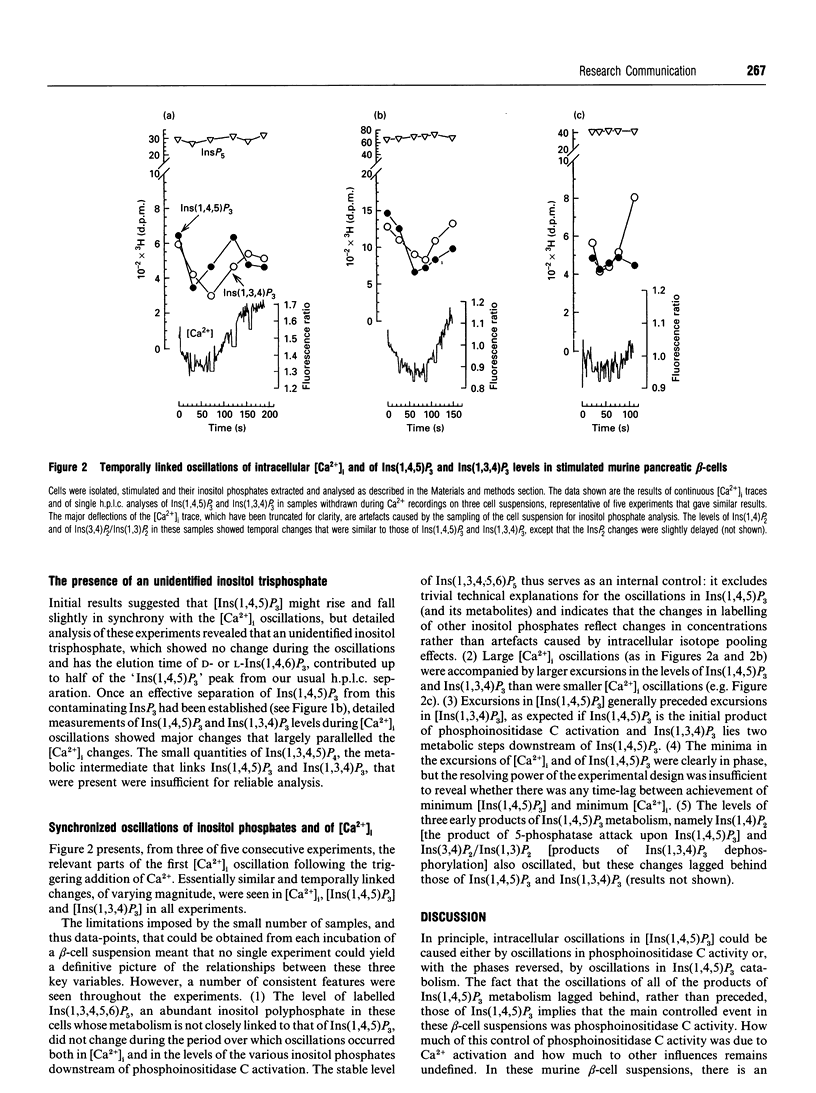
Changes in the cytoplasmic free Ca2+ concentration ([Ca2+]i) in stimulated cells are often oscillatory, but the mechanisms that drive these oscillations are still a matter of controversy: different models of the generation of these [Ca2+]i oscillations make different assumptions as to whether oscillations in Ins(1,4,5)P3 concentration are necessary for this process. We have looked for changes in inositol polyphosphate levels that might occur in suspensions of murine pancreatic beta-cells when these cells are induced to display synchronized oscillations in [Ca2+]i by the sequential addition of glucose, an alpha 2-adrenergic stimulus and extracellular Ca2+. The intracellular level of Ins(1,4,5)P3 oscillated in a manner approximately in synchrony with changes in [Ca2+]i. Oscillations in the levels of Ins(1,4,5)P3 metabolites [Ins(1,3,4)P3 and inositol bisphosphates] were slightly delayed relative to the Ins(1,4,5)P3 oscillations, and the concentration of Ins(1,3,4,5,6)P5 remained approximately constant during the [Ca2+]i oscillations. These results demonstrate that [Ins(1,4,5)P3] and [Ca2+]i oscillate in synchrony in at least one type of cell. Whether such oscillations in intracellular [Ins(1,4,5)P3] provide a primary driving force for [Ca2+]i oscillations either in beta-cells or in other stimulated cells remains to be determined. Even if they do not, the [Ins(1,4,5)P3] oscillations will at least provide an amplifying influence on the [Ca2+]i changes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Hallberg A., Kindmark H., Nilsson T., Rorsman P., Berggren P. O. Extracellular ATP increases cytoplasmic free Ca2+ concentration in clonal insulin-producing RINm5F cells. A mechanism involving direct interaction with both release and refilling of the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. Biochem J. 1990 Jan 1;265(1):203–211. doi: 10.1042/bj2650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium oscillations. Adv Second Messenger Phosphoprotein Res. 1992;26:211–223. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Peter-Riesch B., Schlegel W., Wollheim C. B. Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets. Studies with K+, glucose, and carbamylcholine. J Biol Chem. 1987 Mar 15;262(8):3567–3571. [PubMed] [Google Scholar]
- Cobbold P. H., Sanchez-Bueno A., Dixon C. J. The hepatocyte calcium oscillator. Cell Calcium. 1991 Feb-Mar;12(2-3):87–95. doi: 10.1016/0143-4160(91)90011-3. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hellman B. The significance of calcium for glucose stimulation of insulin release. Endocrinology. 1975 Aug;97(2):392–398. doi: 10.1210/endo-97-2-392. [DOI] [PubMed] [Google Scholar]
- Islam M. S., Berggren P. O. Mobilization of Ca2+ by thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone in permeabilized insulin-secreting RINm5F cells: evidence for separate uptake and release compartments in inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. Biochem J. 1993 Jul 15;293(Pt 2):423–429. doi: 10.1042/bj2930423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindmark H., Köhler M., Efendić S., Rorsman P., Larsson O., Berggren P. O. Protein kinase C activity affects glucose-induced oscillations in cytoplasmic free Ca2+ in the pancreatic B-cell. FEBS Lett. 1992 May 25;303(1):85–90. doi: 10.1016/0014-5793(92)80483-w. [DOI] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Calcium spiking. Annu Rev Biophys Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Berggren P. O. Extracellular Ca2+ induces a rapid increase in cytoplasmic free Ca2+ in pancreatic beta-cells. Biochem Biophys Res Commun. 1987 Nov 30;149(1):152–158. doi: 10.1016/0006-291x(87)91617-2. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Hallberg A., Hellman B., Berggren P. O. Characterization of the inositol 1,4,5-trisphosphate-induced Ca2+ release in pancreatic beta-cells. Biochem J. 1987 Dec 1;248(2):329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Rorsman P., Berggren P. O. Suppression of insulin release by galanin and somatostatin is mediated by a G-protein. An effect involving repolarization and reduction in cytoplasmic free Ca2+ concentration. J Biol Chem. 1989 Jan 15;264(2):973–980. [PubMed] [Google Scholar]
- Rana R. S., Sekar M. C., Hokin L. E., MacDonald M. J. A possible role for glucose metabolites in the regulation of inositol-1,4,5-trisphosphate 5-phosphomonoesterase activity in pancreatic islets. J Biol Chem. 1986 Apr 25;261(12):5237–5240. [PubMed] [Google Scholar]
- Rorsman P., Bokvist K., Ammälä C., Arkhammar P., Berggren P. O., Larsson O., Wåhlander K. Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic B cells. Nature. 1991 Jan 3;349(6304):77–79. doi: 10.1038/349077a0. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Renard D. C., Rooney T. A. Spatial organization of Ca2+ signalling and Ins(1,4,5)P3 action. Adv Second Messenger Phosphoprotein Res. 1992;26:225–263. [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M., Santos R. M., Contreras D., Soria B., Rosario L. M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett. 1989 Dec 18;259(1):19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


