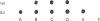Abstract
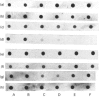
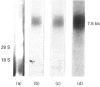
Ultrastructural examination of rat tracheal explants at various times of culture in a serum-free and hormone-supplemented medium containing retinoic acid showed that the cytological characteristics of the epithelium were well preserved for at least 192 h. Hybridization analyses for mucin core protein mRNA in the explants were performed with a 30-base oligonucleotide probe, the design of which was based on the tandem repeat sequence of the rat intestine mucin core protein. The probe reacted with total RNA prepared from trachea, intestine and colon, but not with total RNA obtained from liver or alveolar region of the lung. Type-I keratin expression was observed in the explant grown at different periods of time in a medium with and without retinoic acid. The hybridization probe gave a prominent reaction with RNA preparations obtained from tracheal explants incubated for as long as 192 h in a medium containing retinoic acid. In the absence of retinoic acid, however, the mucin message was evident at the 24 h time point but thereafter decreased to barely detectable levels. When retinoic acid was added at 96 h to the latter cultures, the mucin mRNA was prominent again after additional incubation for 24 and 48 h. Northern-blot analyses of tracheal RNA showed a diffuse band at approx. 7.5 kb. Addition of a variety of chemical and pharmacological agents to explants cultured in the presence of retinoic acid had no dramatic induction or inhibitory effects on the mucin mRNA. Only the steroid prednisolone had a reproducible inhibitory effect.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhargava A. K., Woitach J. T., Davidson E. A., Bhavanandan V. P. Cloning and cDNA sequence of a bovine submaxillary gland mucin-like protein containing two distinct domains. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6798–6802. doi: 10.1073/pnas.87.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Ashbaugh P., Lund M., Manna B. In vitro effects of drugs on production of mucins in rabbit tracheal epithelial cells expressing mucin gene: a model system for studying upper airway respiratory diseases. Inflammation. 1992 Aug;16(4):371–382. doi: 10.1007/BF00917628. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coles S. J., Levine L. R., Reid L. Hypersecretion of mucus glycoproteins in rat airways induced by tobacco smoke. Am J Pathol. 1979 Mar;94(3):459–472. [PMC free article] [PubMed] [Google Scholar]
- Edmondson S. W., Mossman B. T. Alterations in keratin expression in hamster tracheal epithelial cells exposed to benzo[a]pyrene. Carcinogenesis. 1991 Apr;12(4):679–684. doi: 10.1093/carcin/12.4.679. [DOI] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Gerard C., Eddy R. L., Jr, Shows T. B. The core polypeptide of cystic fibrosis tracheal mucin contains a tandem repeat structure. Evidence for a common mucin in airway and gastrointestinal tissue. J Clin Invest. 1990 Dec;86(6):1921–1927. doi: 10.1172/JCI114925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Lagace R. E., Byrd J. C., Toribara N. W., Siddiki B., Fearney F. J., Lamport D. T., Kim Y. S. Molecular cloning of rat intestinal mucin. Lack of conservation between mammalian species. J Biol Chem. 1991 Nov 25;266(33):22733–22738. [PubMed] [Google Scholar]
- Harris C. C., Autrup H., Stoner G. D., McDowell E. M., Trump B. F., Schafer P. Metabolism of acyclic and cyclic N-nitrosamines in cultured human bronchi. J Natl Cancer Inst. 1977 Nov;59(5):1401–1406. doi: 10.1093/jnci/59.5.1401. [DOI] [PubMed] [Google Scholar]
- Huang H. T., Haskell A., McDonald D. M. Changes in epithelial secretory cells and potentiation of neurogenic inflammation in the trachea of rats with respiratory tract infections. Anat Embryol (Berl) 1989;180(4):325–341. doi: 10.1007/BF00311165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jany B., Basbaum C. B. Mucin in disease. Modification of mucin gene expression in airway disease. Am Rev Respir Dis. 1991 Sep;144(3 Pt 2):S38–S41. doi: 10.1164/ajrccm/144.3_pt_2.S38. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. M. The effect of tobacco smoke, with or without phenylmethyloxadiazole (PMO), on rat bronchial epithelium: a light and electron microscopic study. J Pathol. 1981 Apr;133(4):341–359. doi: 10.1002/path.1711330406. [DOI] [PubMed] [Google Scholar]
- Krieg T. M., Schafer M. P., Cheng C. K., Filpula D., Flaherty P., Steinert P. M., Roop D. R. Organization of a type I keratin gene. Evidence for evolution of intermediate filaments from a common ancestral gene. J Biol Chem. 1985 May 25;260(10):5867–5870. [PubMed] [Google Scholar]
- Lamb D., Reid L. Goblet cell increase in rat bronchial epithelium after exposure to cigarette and cigar tobacco smoke. Br Med J. 1969 Jan 4;1(5635):33–35. doi: 10.1136/bmj.1.5635.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M. S., Batra S. K., Qi W. N., Metzgar R. S., Hollingsworth M. A. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem. 1990 Sep 5;265(25):15294–15299. [PubMed] [Google Scholar]
- Plopper C. G., Mariassy A. T., Wilson D. W., Alley J. L., Nishio S. J., Nettesheim P. Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res. 1983 Dec;5(4):281–294. doi: 10.3109/01902148309061521. [DOI] [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Rearick J. I., Deas M., Jetten A. M. Synthesis of mucous glycoproteins by rabbit tracheal cells in vitro. Modulation by substratum, retinoids and cyclic AMP. Biochem J. 1987 Feb 15;242(1):19–25. doi: 10.1042/bj2420019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H. L., Floyd E. E., Jetten A. M. Molecular cloning of gene sequences regulated during squamous differentiation of tracheal epithelial cells and controlled by retinoic acid. Mol Cell Biol. 1987 Nov;7(11):4017–4023. doi: 10.1128/mcb.7.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer A. P., Parry G., Patton S., Gendler S. J. Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. J Biol Chem. 1991 Aug 15;266(23):15099–15109. [PubMed] [Google Scholar]
- Timpte C. S., Eckhardt A. E., Abernethy J. L., Hill R. L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J Biol Chem. 1988 Jan 15;263(2):1081–1088. [PubMed] [Google Scholar]