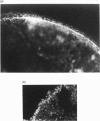
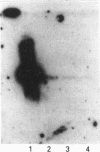
Abstract
In the presence of 150 microM BSA, uptake of [3H]oleate by Xenopus laevis oocytes was a saturable function of the unbound oleate concentration (Vmax. 110 +/- 4 pmol/h per oocyte; Km 193 +/- 11 nM unbound oleate). Oleate uptake was three orders of magnitude faster than that of another test substance, [35S]bromosulphophthalein, and was competitively inhibited by 55 nM unbound palmitate (Vmax. 111 +/- 14 pmol/h per oocyte; Km 424 +/- 63 nM unbound oleate) (P < 0.01). Oleate uptake was also inhibited by antibodies to a 43 kDa rat liver plasma-membrane fatty acid-binding protein, a putative transporter of long-chain fatty acids in mammalian cells; uptake of the medium-chain fatty acid [14C]octanoate was unaffected. Immunofluorescence and immunoblotting demonstrated that the antiserum reacted with a single 43 kDa protein on the oocyte surface. Hence a protein related to the mammalian plasma-membrane fatty acid-binding protein may play a role in saturable uptake of long-chain fatty acids by Xenopus oocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Ananthanarayanan M., von Dippe P., Levy D. Identification of the hepatocyte Na+-dependent bile acid transport protein using monoclonal antibodies. J Biol Chem. 1988 Jun 15;263(17):8338–8343. [PubMed] [Google Scholar]
- Berk P. D., Wada H., Horio Y., Potter B. J., Sorrentino D., Zhou S. L., Isola L. M., Stump D., Kiang C. L., Thung S. Plasma membrane fatty acid-binding protein and mitochondrial glutamic-oxaloacetic transaminase of rat liver are related. Proc Natl Acad Sci U S A. 1990 May;87(9):3484–3488. doi: 10.1073/pnas.87.9.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N., Said B., Ghosn C. R., Beach J. V., Nunn W. D. Purification and characterization of an outer membrane-bound protein involved in long-chain fatty acid transport in Escherichia coli. J Biol Chem. 1987 Jan 25;262(3):1412–1419. [PubMed] [Google Scholar]
- Boyer J. L., Hagenbuch B., Ananthanarayanan M., Suchy F., Stieger B., Meier P. J. Phylogenic and ontogenic expression of hepatocellular bile acid transport. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):435–438. doi: 10.1073/pnas.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. E., Ashbrook J. D., Spector A. A. Computer analysis of drug-protein binding data. Ann N Y Acad Sci. 1973 Nov 26;226:69–81. doi: 10.1111/j.1749-6632.1973.tb20469.x. [DOI] [PubMed] [Google Scholar]
- Fletcher J. E., Spector A. A., Ashbrook J. D. Analysis of macromolecule--ligand binding by determination of stepwise equilibrium constants. Biochemistry. 1970 Nov 10;9(23):4580–4587. doi: 10.1021/bi00825a018. [DOI] [PubMed] [Google Scholar]
- Fricker G., Hugentobler G., Meier P. J., Kurz G., Boyer J. L. Identification of a single sinusoidal bile salt uptake system in skate liver. Am J Physiol. 1987 Dec;253(6 Pt 1):G816–G822. doi: 10.1152/ajpgi.1987.253.6.G816. [DOI] [PubMed] [Google Scholar]
- Glatz J. F., van der Vusse G. J. Cellular fatty acid-binding proteins: current concepts and future directions. 1990 Oct 15-Nov 8Mol Cell Biochem. 98(1-2):237–251. doi: 10.1007/BF00231390. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B., Stieger B., Foguet M., Lübbert H., Meier P. J. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon C. M., Luce P., Beth A. H., Abumrad N. A. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol. 1991 May;121(3):261–268. doi: 10.1007/BF01951559. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl W. E., Spector A. A. Uptake of long-chain fatty acid methyl esters by mammalian cells. J Lipid Res. 1970 Sep;11(5):458–465. [PubMed] [Google Scholar]
- Kurisu H., Nilprabhassorn P., Wolkoff A. W. Preparation of [35S]sulfobromophthalein of high specific activity. Anal Biochem. 1989 May 15;179(1):72–74. doi: 10.1016/0003-2697(89)90202-9. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Colburn R. W., Black P. N. Transport of long-chain fatty acids in Escherichia coli. Evidence for role of fadL gene product as long-chain fatty acid receptor. J Biol Chem. 1986 Jan 5;261(1):167–171. [PubMed] [Google Scholar]
- Potter B. J., Stump D., Schwieterman W., Sorrentino D., Jacobs L. N., Kiang C. L., Rand J. H., Berk P. D. Isolation and partial characterization of plasma membrane fatty acid binding proteins from myocardium and adipose tissue and their relationship to analogous proteins in liver and gut. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1370–1376. doi: 10.1016/s0006-291x(87)80283-8. [DOI] [PubMed] [Google Scholar]
- Said B., Ghosn C. R., Vu L., Nunn W. D. Nucleotide sequencing and expression of the fadL gene involved in long-chain fatty acid transport in Escherichia coli. Mol Microbiol. 1988 May;2(3):363–370. doi: 10.1111/j.1365-2958.1988.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Grossbard M., Gordon E. R., Boyer J. L. Taurocholate uptake by isolated skate hepatocytes: effect of albumin. Am J Physiol. 1987 Apr;252(4 Pt 1):G479–G484. doi: 10.1152/ajpgi.1987.252.4.G479. [DOI] [PubMed] [Google Scholar]
- Sorrentino D., Potter B. J., Berk P. D. From albumin to the cytoplasm: the hepatic uptake of organic anions. Prog Liver Dis. 1990;9:203–224. [PubMed] [Google Scholar]
- Sorrentino D., Stump D., Potter B. J., Robinson R. B., White R., Kiang C. L., Berk P. D. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J Clin Invest. 1988 Sep;82(3):928–935. doi: 10.1172/JCI113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino D., Zhou S. L., Kokkotou E., Berk P. D. Sex differences in hepatic fatty acid uptake reflect a greater affinity of the transport system in females. Am J Physiol. 1992 Sep;263(3 Pt 1):G380–G385. doi: 10.1152/ajpgi.1992.263.3.G380. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci U S A. 1986 May;83(10):3086–3090. doi: 10.1073/pnas.83.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Theilmann L. Selective inhibition of long-chain fatty acid uptake in short-term cultured rat hepatocytes by an antibody to the rat liver plasma membrane fatty acid-binding protein. Biochim Biophys Acta. 1986 Jun 11;877(1):191–197. doi: 10.1016/0005-2760(86)90134-7. [DOI] [PubMed] [Google Scholar]
- Stremmel W. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J Clin Invest. 1988 Dec;82(6):2001–2010. doi: 10.1172/JCI113820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump D. D., Nunes R. M., Sorrentino D., Isola L. M., Berk P. D. Characteristics of oleate binding to liver plasma membranes and its uptake by isolated hepatocytes. J Hepatol. 1992 Nov;16(3):304–315. doi: 10.1016/s0168-8278(05)80661-0. [DOI] [PubMed] [Google Scholar]
- Swick A. G., Janicot M., Cheneval-Kastelic T., McLenithan J. C., Lane M. D. Promoter-cDNA-directed heterologous protein expression in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1812–1816. doi: 10.1073/pnas.89.5.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnuzzer R. W., Campa M. J., Qian N. X., Englesberg E., Kilberg M. S. Expression of the mammalian system A neutral amino acid transporter in Xenopus oocytes. J Biol Chem. 1990 Aug 15;265(23):13914–13917. [PubMed] [Google Scholar]
- Trigatti B. L., Mangroo D., Gerber G. E. Photoaffinity labeling and fatty acid permeation in 3T3-L1 adipocytes. J Biol Chem. 1991 Nov 25;266(33):22621–22625. [PubMed] [Google Scholar]
- Van 't Klooster G. A., Boot J. H., Mennes W. C., Blaauboer B. J. Rapid method for the determination and quantification of bromosulphophthalein and metabolites in cultured hepatocytes, culture media and bile by high-performance liquid chromatography. J Chromatogr. 1988 Nov 18;432:223–231. doi: 10.1016/s0378-4347(00)80647-7. [DOI] [PubMed] [Google Scholar]
- Wieland T., Nassal M., Kramer W., Fricker G., Bickel U., Kurz G. Identity of hepatic membrane transport systems for bile salts, phalloidin, and antamanide by photoaffinity labeling. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5232–5236. doi: 10.1073/pnas.81.16.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosilait W. D., Nagy P. A method of computing drug distribution in plasma using stepwise association constants: clofibrate acid as an illustrative example. Comput Programs Biomed. 1976 Oct;6(3):142–148. doi: 10.1016/0010-468x(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Zhou S. L., Stump D., Sorrentino D., Potter B. J., Berk P. D. Adipocyte differentiation of 3T3-L1 cells involves augmented expression of a 43-kDa plasma membrane fatty acid-binding protein. J Biol Chem. 1992 Jul 15;267(20):14456–14461. [PubMed] [Google Scholar]
- von Dippe P., Levy D. Expression of the bile acid transport protein during liver development and in hepatoma cells. J Biol Chem. 1990 Apr 15;265(11):5942–5945. [PubMed] [Google Scholar]