Abstract
Evidence has accumulated that cyclic AMP (cAMP)-induced phosphorylation of a Ras-related protein (Rap1) regulates platelet Ca2+ transport. As this transport was recently found to be controlled by two isoforms of sarcoendoplasmic reticulum Ca(2+)-ATPase (SERCA), the 100 kDa SERCA2b and the newly identified 97 kDa SERCA, we attempted to establish which isoform is involved in this regulation. For this purpose, we studied the expression and regulation of both the SERCA and Rap1 isoforms in platelets, haemopoietic cells and various cancer cell lines. SERCA2b was shown to be equally expressed in all the cell lines tested, as determined by detection of its phosphoenzyme formation and by Western blotting using an isoform-specific antibody. In contrast, the expression of the 97 kDa SERCA, studied by the same methods, varied from total absence in the cancer cells to high levels in the megakaryocytic cell lines. With regard to the potential regulatory Rap1 proteins, Western blotting showed different expression of total Rap1 isoforms among the cell lineages, thus ruling out any possible relationship between Rap1 and SERCA2b. However, the expression of Rap1 proteins correlated with that of the 97 kDa SERCA isoform. More refined analysis of the rap1A and rap1B isoforms by reverse transcription PCR and by determining cAMP-induced phosphorylation of Rap1B, i.e. its functional mechanism, confirmed the correlation between Rap1B and the 97 kDa SERCA expression. This relationship was also established by the concerted up-regulation of these two proteins demonstrated in the pathological model of platelets from hypertensive rats. It is concluded that the expressions of 97 KDa SERCA and Rap1B are related, suggesting that regulation of the platelet Ca(2+)-ATPase system by cAMP-induced phosphorylation of Rap1B specifically involves the 97 kDa SERCA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adunyah S. E., Dean W. L. Inositol triphosphate-induced Ca2+ release from human platelet membranes. Biochem Biophys Res Commun. 1985 May 16;128(3):1274–1280. doi: 10.1016/0006-291x(85)91078-2. [DOI] [PubMed] [Google Scholar]
- Adunyah S. E., Dean W. L. Regulation of human platelet membrane Ca2+ transport by cAMP- and calmodulin-dependent phosphorylation. Biochim Biophys Acta. 1987 Oct 1;930(3):401–409. doi: 10.1016/0167-4889(87)90013-9. [DOI] [PubMed] [Google Scholar]
- Authi K. S., Crawford N. Inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ from highly purified human platelet intracellular membranes. Biochem J. 1985 Aug 15;230(1):247–253. doi: 10.1042/bj2300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar R. P., Haslam R. J. Gn-proteins are distinct from ras p21 and other known low molecular mass GTP-binding proteins in the platelet. FEBS Lett. 1988 Sep 12;237(1-2):168–172. doi: 10.1016/0014-5793(88)80194-7. [DOI] [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Béranger F., Goud B., Tavitian A., de Gunzburg J. Association of the Ras-antagonistic Rap1/Krev-1 proteins with the Golgi complex. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1606–1610. doi: 10.1073/pnas.88.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. The Ca2+ pump of the plasma membrane. J Biol Chem. 1992 Feb 5;267(4):2115–2118. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Corvazier E., Enouf J., Papp B., de Gunzburg J., Tavitian A., Levy-Toledano S. Evidence for a role of rap1 protein in the regulation of human platelet Ca2+ fluxes. Biochem J. 1992 Jan 15;281(Pt 2):325–331. doi: 10.1042/bj2810325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culine S., Olofsson B., Gosselin S., Honore N., Tavitian A. Expression of the ras-related rap genes in human tumors. Int J Cancer. 1989 Dec 15;44(6):990–994. doi: 10.1002/ijc.2910440608. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., Verbist J., Casteels R. Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J. 1990 Nov 1;271(3):649–653. doi: 10.1042/bj2710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enouf J., Bredoux R., Boucheix C., Mirshahi M., Soria C., Levy-Toledano S. Possible involvement of two proteins (phosphoprotein and CD9 (p24)) in regulation of platelet calcium fluxes. FEBS Lett. 1985 Apr 22;183(2):398–402. doi: 10.1016/0014-5793(85)80819-x. [DOI] [PubMed] [Google Scholar]
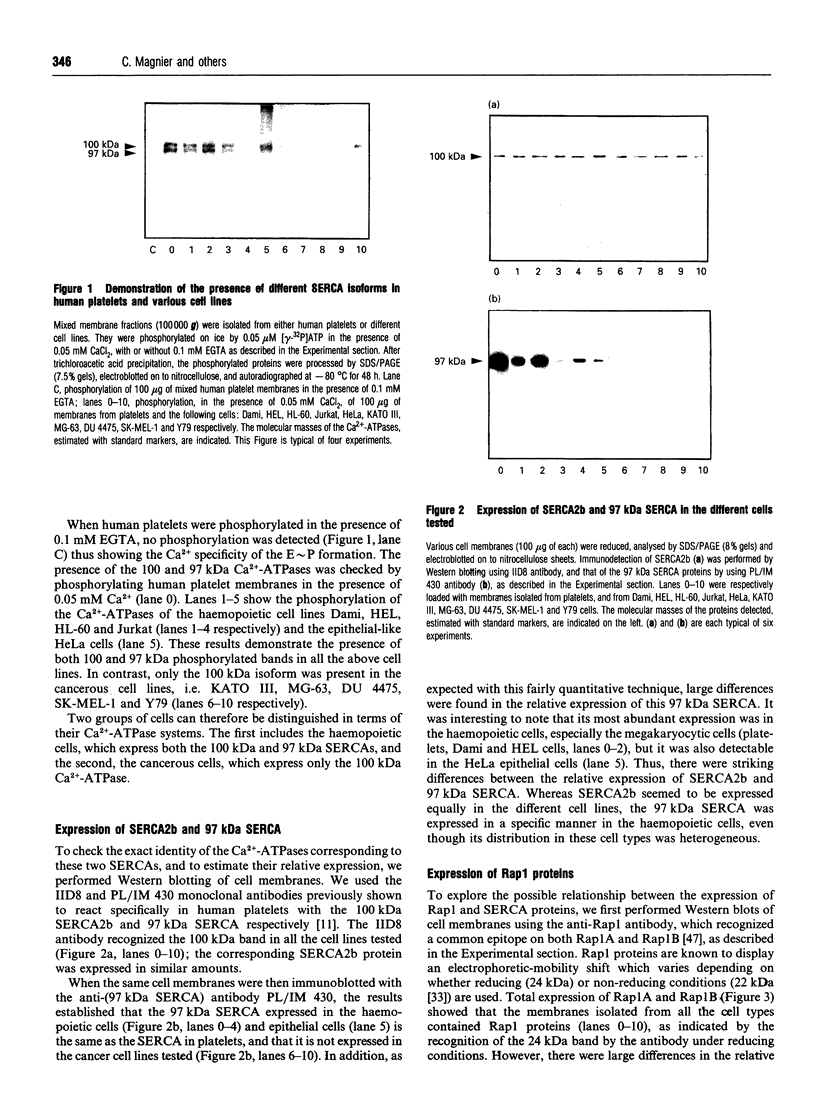
- Enouf J., Bredoux R., Papp B., Djaffar I., Lompré A. M., Kieffer N., Gayet O., Clemetson K., Wuytack F., Rosa J. P. Human platelets express the SERCA2-b isoform of Ca(2+)-transport ATPase. Biochem J. 1992 Aug 15;286(Pt 1):135–140. doi: 10.1042/bj2860135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enouf J., Lévy-Toledano S. Relationship between structure of phenothiazine analogues and their activity on platelet calcium fluxes. Br J Pharmacol. 1984 Mar;81(3):509–518. doi: 10.1111/j.1476-5381.1984.tb10104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B. Release of intracellular membrane-bound calcium precedes the onset of stimulus-induced exocytosis in platelets. Biochem Biophys Res Commun. 1980 Mar 28;93(2):593–600. doi: 10.1016/0006-291x(80)91119-5. [DOI] [PubMed] [Google Scholar]
- Fischer T. H., Gatling M. N., Lacal J. C., White G. C., 2nd rap1B, a cAMP-dependent protein kinase substrate, associates with the platelet cytoskeleton. J Biol Chem. 1990 Nov 15;265(32):19405–19408. [PubMed] [Google Scholar]
- Fischer T. H., White G. C., 2nd cAMP-dependent protein kinase substrates in platelets. Evidence that thrombolamban, a 22,000 dalton substrate, and the Ca++-ATPase are not associated proteins. Biochem Biophys Res Commun. 1989 Mar 15;159(2):644–650. doi: 10.1016/0006-291x(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Say A. K., Haslam R. J. Subcellular distribution of the different platelet proteins phosphorylated on exposure of intact platelets to ionophore A23187 or to prostaglandin E1. Possible role of a membrane phosphopolypeptide in the regulation of calcium-ion transport. Biochem J. 1979 Dec 15;184(3):651–661. doi: 10.1042/bj1840651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. M., Rosenthal D. S., Greeley T. A., Tantravahi R., Handin R. I. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988 Dec;72(6):1968–1977. [PubMed] [Google Scholar]
- Hack N., Wilkinson J. M., Crawford N. A monoclonal antibody (PL/IM 430) to human platelet intracellular membranes which inhibits the uptake of Ca2+ without affecting the Ca2+ +Mg2+-ATPase. Biochem J. 1988 Mar 1;250(2):355–361. doi: 10.1042/bj2500355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Hoshijima M., Kikuchi A., Kawata M., Ohmori T., Hashimoto E., Yamamura H., Takai Y. Phosphorylation by cyclic AMP-dependent protein kinase of a human platelet Mr 22,000 GTP-binding protein (smg p21) having the same putative effector domain as the ras gene products. Biochem Biophys Res Commun. 1988 Dec 30;157(3):851–860. doi: 10.1016/s0006-291x(88)80953-7. [DOI] [PubMed] [Google Scholar]
- James P., Inui M., Tada M., Chiesi M., Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989 Nov 2;342(6245):90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Johansson J. S., Nied L. E., Haynes D. H. Cyclic AMP stimulates Ca(2+)-ATPase-mediated Ca2+ extrusion from human platelets. Biochim Biophys Acta. 1992 Mar 23;1105(1):19–28. doi: 10.1016/0005-2736(92)90158-i. [DOI] [PubMed] [Google Scholar]
- Kawata M., Matsui Y., Kondo J., Hishida T., Teranishi Y., Takai Y. A novel small molecular weight GTP-binding protein with the same putative effector domain as the ras proteins in bovine brain membranes. Purification, determination of primary structure, and characterization. J Biol Chem. 1988 Dec 15;263(35):18965–18971. [PubMed] [Google Scholar]
- Klinz F. J., Seifert R., Schwaner I., Gausepohl H., Frank R., Schultz G. Generation of specific antibodies against the rap1A, rap1B and rap2 small GTP-binding proteins. Analysis of rap and ras proteins in membranes from mammalian cells. Eur J Biochem. 1992 Jul 1;207(1):207–213. doi: 10.1111/j.1432-1033.1992.tb17039.x. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Lacal J. C., Reep B. R., Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci U S A. 1989 May;86(9):3131–3134. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B. R. Specific binding of [alpha-32P]GTP to cytosolic and membrane-bound proteins of human platelets correlates with the activation of phospholipase C. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2261–2265. doi: 10.1073/pnas.84.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Härtner K. T., Brandl C. J., Fujii J., Tada M., MacLennan D. H., Pette D. Slow/cardiac sarcoplasmic reticulum Ca2+-ATPase and phospholamban mRNAs are expressed in chronically stimulated rabbit fast-twitch muscle. Eur J Biochem. 1989 Oct 20;185(1):51–54. doi: 10.1111/j.1432-1033.1989.tb15080.x. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Clarke D. M., Fujii J., Loo T. W., MacLennan D. H. Expression and mutation of Ca2+ ATPases of the sarcoplasmic reticulum. Cell Motil Cytoskeleton. 1989;14(1):26–34. doi: 10.1002/cm.970140107. [DOI] [PubMed] [Google Scholar]
- Nagai R., Zarain-Herzberg A., Brandl C. J., Fujii J., Tada M., MacLennan D. H., Alpert N. R., Periasamy M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Nagao S., Nozawa Y. Low Mr GTP-binding proteins in human platelets: cyclic AMP-dependent protein kinase phosphorylates m22KG(I) in membrane but not c21KG in cytosol. Biochem Biophys Res Commun. 1989 Apr 14;160(1):235–242. doi: 10.1016/0006-291x(89)91646-x. [DOI] [PubMed] [Google Scholar]
- Nagata K., Nozawa Y. Purification and characterization of two GTP-binding proteins of 22 kDa from human platelet membranes. FEBS Lett. 1988 Sep 26;238(1):90–94. doi: 10.1016/0014-5793(88)80232-1. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- O'Rourke F., Zavoico G. B., Feinstein M. B. Release of Ca2+ by inositol 1,4,5-trisphosphate in platelet membrane vesicles is not dependent on cyclic AMP-dependent protein kinase. Biochem J. 1989 Feb 1;257(3):715–721. doi: 10.1042/bj2570715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori T., Kikuchi A., Yamamoto K., Kim S., Takai Y. Small molecular weight GTP-binding proteins in human platelet membranes. Purification and characterization of a novel GTP-binding protein with a molecular weight of 22,000. J Biol Chem. 1989 Jan 25;264(3):1877–1881. [PubMed] [Google Scholar]
- Papp B., Corvazier E., Magnier C., Kovàcs T., Bourdeau N., Lévy-Tolédano S., Bredoux R., Lévy B., Poitevin P., Lompré A. M. Spontaneously hypertensive rats and platelet Ca(2+)-ATPases: specific up-regulation of the 97 kDa isoform. Biochem J. 1993 Nov 1;295(Pt 3):685–690. doi: 10.1042/bj2950685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp B., Enyedi A., Kovács T., Sarkadi B., Wuytack F., Thastrup O., Gárdos G., Bredoux R., Levy-Toledano S., Enouf J. Demonstration of two forms of calcium pumps by thapsigargin inhibition and radioimmunoblotting in platelet membrane vesicles. J Biol Chem. 1991 Aug 5;266(22):14593–14596. [PubMed] [Google Scholar]
- Papp B., Enyedi A., Pászty K., Kovács T., Sarkadi B., Gárdos G., Magnier C., Wuytack F., Enouf J. Simultaneous presence of two distinct endoplasmic-reticulum-type calcium-pump isoforms in human cells. Characterization by radio-immunoblotting and inhibition by 2,5-di-(t-butyl)-1,4-benzohydroquinone. Biochem J. 1992 Nov 15;288(Pt 1):297–302. doi: 10.1042/bj2880297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V., Chardin P., Lerosey I., Olofsson B., Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the 'effector' region. Oncogene. 1988 Aug;3(2):201–204. [PubMed] [Google Scholar]
- Pizon V., Lerosey I., Chardin P., Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B). Nucleic Acids Res. 1988 Aug 11;16(15):7719–7719. doi: 10.1093/nar/16.15.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers L., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J. 1988 May 15;252(1):269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Functional relationship between cyclic AMP-dependent protein phosphorylation and platelet inhibition. Biochem J. 1990 Nov 1;271(3):815–819. doi: 10.1042/bj2710815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W., Winegar D. A., Lapetina E. G. Rap1-B is phosphorylated by protein kinase A in intact human platelets. Biochem Biophys Res Commun. 1990 Jul 31;170(2):944–950. doi: 10.1016/0006-291x(90)92182-y. [DOI] [PubMed] [Google Scholar]
- Spencer G. G., Yu X. H., Khan I., Grover A. K. Expression of isoforms of internal Ca2+ pump in cardiac, smooth muscle and non-muscle tissues. Biochim Biophys Acta. 1991 Mar 18;1063(1):15–20. doi: 10.1016/0005-2736(91)90347-b. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Salzman E. W. Cyclic nucleotides in hemostasis and thrombosis. Adv Cyclic Nucleotide Res. 1980;12:71–92. [PubMed] [Google Scholar]
- Tada M., Katz A. M. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- Tao J., Johansson J. S., Haynes D. H. Stimulation of dense tubular Ca2+ uptake in human platelets by cAMP. Biochim Biophys Acta. 1992 Mar 23;1105(1):29–39. doi: 10.1016/0005-2736(92)90159-j. [DOI] [PubMed] [Google Scholar]
- Torti M., Lapetina E. G. Role of rap1B and p21ras GTPase-activating protein in the regulation of phospholipase C-gamma 1 in human platelets. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7796–7800. doi: 10.1073/pnas.89.16.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., Kurzydlowski K., Tada M., MacLennan D. H. Identification of regions in the Ca(2+)-ATPase of sarcoplasmic reticulum that affect functional association with phospholamban. J Biol Chem. 1993 Feb 5;268(4):2809–2815. [PubMed] [Google Scholar]
- White G. C., 2nd, Barton D. W., White T. E., Fischer T. H. Cyclic AMP-dependent protein kinase does not increase calcium transport in platelet microsomes. Thromb Res. 1989 Dec 1;56(5):575–581. doi: 10.1016/0049-3848(89)90265-x. [DOI] [PubMed] [Google Scholar]
- White T. E., Lacal J. C., Reep B., Fischer T. H., Lapetina E. G., White G. C., 2nd Thrombolamban, the 22-kDa platelet substrate of cyclic AMP-dependent protein kinase, is immunologically homologous with the Ras family of GTP-binding proteins. Proc Natl Acad Sci U S A. 1990 Jan;87(2):758–762. doi: 10.1073/pnas.87.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]