Abstract
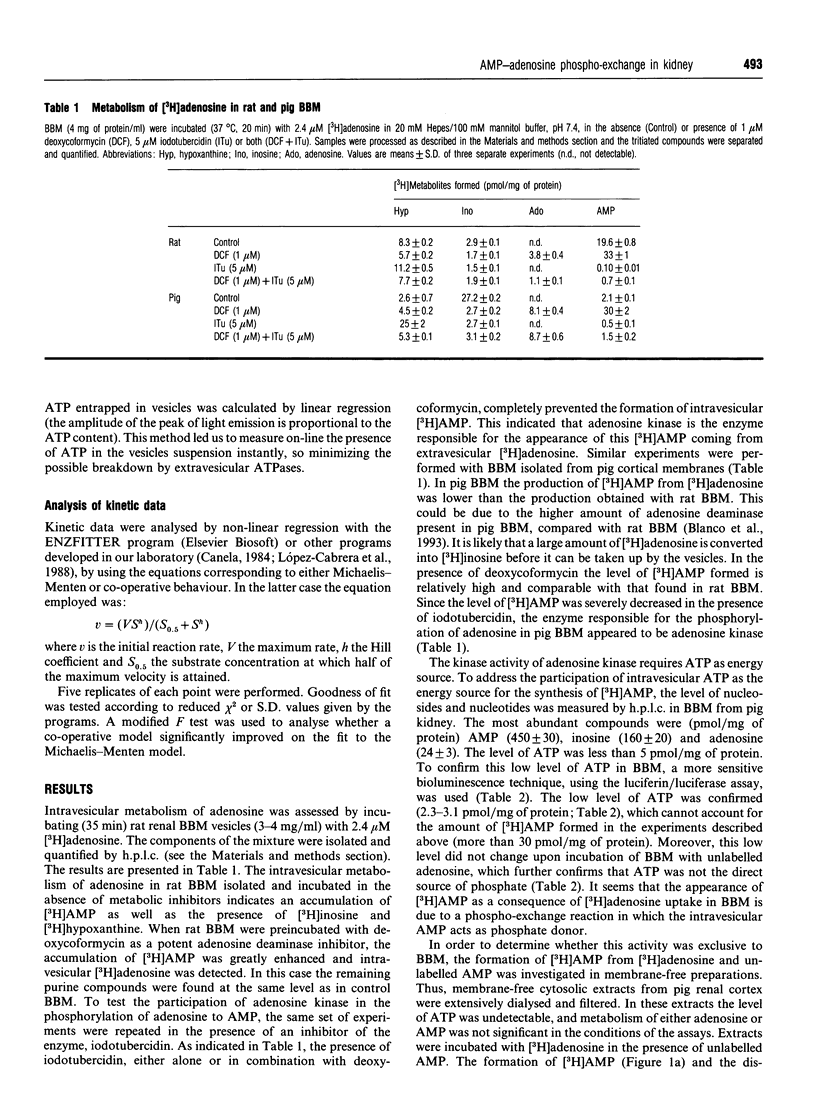
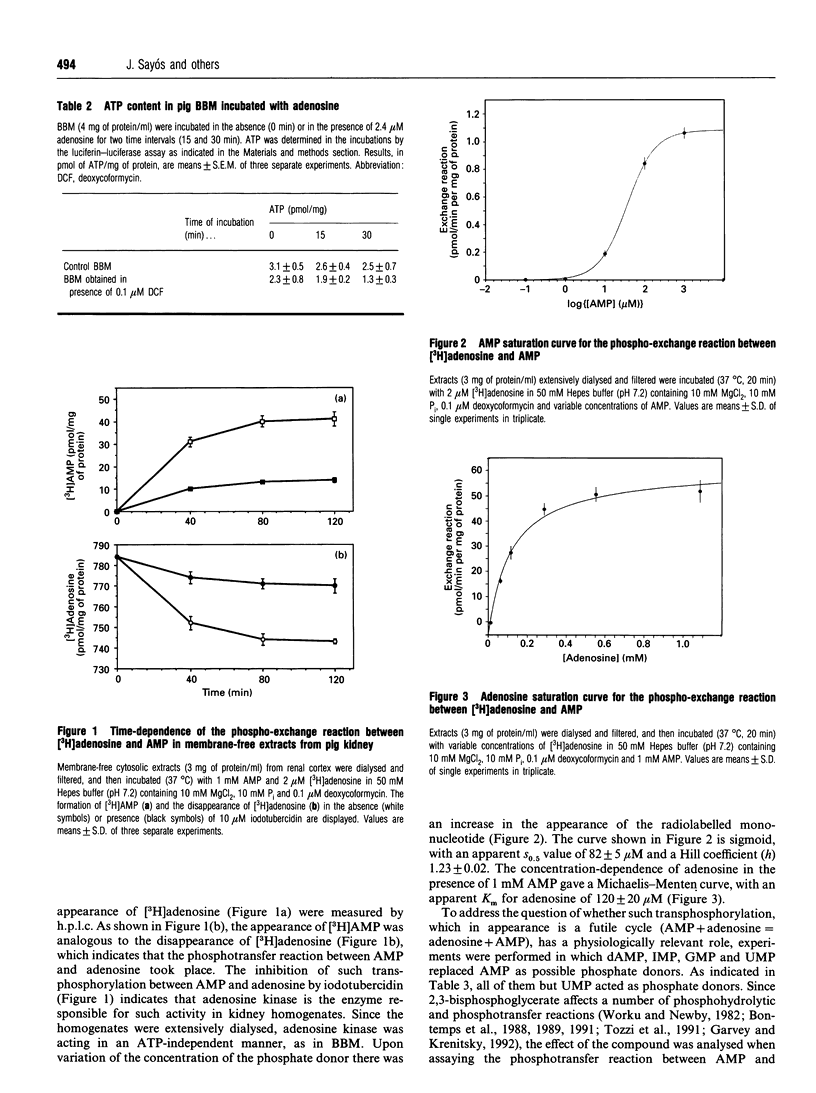
Uptake of [3H]adenosine in brush-border membrane (BBM) vesicles from either rat or pig kidney leads to an accumulation of intravesicular [3H]AMP. The lack of significant levels of ATP and the presence of AMP in BBM indicated that a phosphotransfer between [3H]adenosine and AMP occurs. The phosphotransfer activity is inhibited by iodotubercidin, which suggests that it is performed by adenosine kinase acting in an ATP-independent manner. The existence of a similar phosphotransferase activity was demonstrated in membrane-free extracts from pig kidney. From the compounds tested it was shown that a variety of mononucleotides could act as phosphate donors. The results suggest that phosphotransfer reactions may be physiologically relevant in kidney.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend L. J., Burnatowska-Hledin M. A., Spielman W. S. Adenosine receptor-mediated calcium mobilization in cortical collecting tubule cells. Am J Physiol. 1988 Nov;255(5 Pt 1):C581–C588. doi: 10.1152/ajpcell.1988.255.5.C581. [DOI] [PubMed] [Google Scholar]
- Blanco J., Canela E. I., Mallol J., Lluís C., Franco R. Characterization of adenosine receptors in brush-border membranes from pig kidney. Br J Pharmacol. 1992 Nov;107(3):671–678. doi: 10.1111/j.1476-5381.1992.tb14505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J., Canela E. I., Sayós J., Mallol J., Lluis C., Franco R. Adenine nucleotides and adenosine metabolism in pig kidney proximal tubule membranes. J Cell Physiol. 1993 Oct;157(1):77–83. doi: 10.1002/jcp.1041570110. [DOI] [PubMed] [Google Scholar]
- Blanco J., Mallol J., Lluis C., Canela E. I., Franco R. Adenosine metabolism in kidney slices under normoxic conditions. J Cell Physiol. 1990 May;143(2):344–351. doi: 10.1002/jcp.1041430219. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Mimouni M., Van den Berghe G. Phosphorylation of adenosine by an exchange reaction between AMP and adenosine in anoxic hepatocytes. Adv Exp Med Biol. 1991;309A:317–320. doi: 10.1007/978-1-4899-2638-8_72. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Mimouni M., Van den Berghe G. Phosphorylation of adenosine in anoxic hepatocytes by an exchange reaction catalysed by adenosine kinase. Biochem J. 1993 Mar 15;290(Pt 3):679–684. doi: 10.1042/bj2900679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. 5'-Nucleotidase activities in human erythrocytes. Identification of a purine 5'-nucleotidase stimulated by ATP and glycerate 2,3-bisphosphate. Biochem J. 1988 Mar 15;250(3):687–696. doi: 10.1042/bj2500687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Vincent M. F., Van den Bergh F., van Waeg G., Van den Berghe G. Stimulation by glycerate 2,3-bisphosphate: a common property of cytosolic IMP-GMP 5'-nucleotidase in rat and human tissues. Biochim Biophys Acta. 1989 Jul 27;997(1-2):131–134. doi: 10.1016/0167-4838(89)90144-1. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Vincent M. F., Van den Berghe G. Mechanisms of elevation of adenosine levels in anoxic hepatocytes. Biochem J. 1993 Mar 15;290(Pt 3):671–677. doi: 10.1042/bj2900671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadnapaphornchai P., Kellner D., Golembieski A., McDonald F. D. Roles of adenosine and theophylline on the recovery of adenine nucleotides in postischemic cultured renal tubular cells. J Pharmacol Exp Ther. 1991 May;257(2):774–780. [PubMed] [Google Scholar]
- Canela E. I. A free derivative program for non-linear regression analysis of enzyme kinetics to be used on small computers. Int J Biomed Comput. 1984 Mar-Apr;15(2):121–130. doi: 10.1016/0020-7101(84)90024-2. [DOI] [PubMed] [Google Scholar]
- Culić O., Sabolić I., Zanić-Grubisić T. The stepwise hydrolysis of adenine nucleotides by ectoenzymes of rat renal brush-border membranes. Biochim Biophys Acta. 1990 Nov 30;1030(1):143–151. doi: 10.1016/0005-2736(90)90249-n. [DOI] [PubMed] [Google Scholar]
- Flessner M. F., Wall S. M., Knepper M. A. Ammonium and bicarbonate transport in rat outer medullary collecting ducts. Am J Physiol. 1992 Jan;262(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1992.262.1.F1. [DOI] [PubMed] [Google Scholar]
- Franco R., Centelles J. J., Kinne R. K. Further characterization of adenosine transport in renal brush-border membranes. Biochim Biophys Acta. 1990 May 24;1024(2):241–248. doi: 10.1016/0005-2736(90)90350-w. [DOI] [PubMed] [Google Scholar]
- Freissmuth M., Hausleithner V., Tuisl E., Nanoff C., Schütz W. Glomeruli and microvessels of the rabbit kidney contain both A1- and A2-adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):438–444. doi: 10.1007/BF00165560. [DOI] [PubMed] [Google Scholar]
- Freissmuth M., Nanoff C., Tuisl E., Schuetz W. Stimulation of adenylate cyclase activity via A2-adenosine receptors in isolated tubules of the rabbit renal cortex. Eur J Pharmacol. 1987 Jun 12;138(1):137–140. doi: 10.1016/0014-2999(87)90350-5. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Krenitsky T. A. A novel human phosphotransferase highly specific for adenosine. Arch Biochem Biophys. 1992 Jul;296(1):161–169. doi: 10.1016/0003-9861(92)90558-e. [DOI] [PubMed] [Google Scholar]
- Hammer D. F., Unverferth D. V., Kelley R. E., Harvan P. A., Altschuld R. A. Extraction and measurement of myocardial nucleotides, nucleosides, and purine bases by high-performance liquid chromatography. Anal Biochem. 1988 Mar;169(2):300–305. doi: 10.1016/0003-2697(88)90288-6. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Dubach U. C. Sodium gradient-energized concentrative transport of adenosine in renal brush border vesicles. Pflugers Arch. 1984 May;401(1):58–63. doi: 10.1007/BF00581533. [DOI] [PubMed] [Google Scholar]
- Lin J. T., Da Cruz M. E., Riedel S., Kinne R. Partial purification of hog kidney sodium-D-glucose cotransport system by affinity chromatography on a phlorizin polymer. Biochim Biophys Acta. 1981 Jan 8;640(1):43–54. doi: 10.1016/0005-2736(81)90530-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Cabrera A., Cabré F., Franco R., Canela E. I. Identification and rejection of outliers in enzyme kinetics. Int J Biomed Comput. 1988 Oct;23(1-2):9–20. doi: 10.1016/0020-7101(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Takano T., Soltoff S. P., Murdaugh S. Mechanisms whereby exogenous adenine nucleotides improve rabbit renal proximal function during and after anoxia. J Clin Invest. 1988 Apr;81(4):1255–1264. doi: 10.1172/JCI113443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghji P., Middleton K. M., Newby A. C. Absolute rates of adenosine formation during ischaemia in rat and pigeon hearts. Biochem J. 1988 Feb 1;249(3):695–703. doi: 10.1042/bj2490695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. D., Churchill P. C. Concentration dependency of the renal vascular and renin secretory responses to adenosine receptor agonists. J Pharmacol Exp Ther. 1985 Jan;232(1):189–193. [PubMed] [Google Scholar]
- Olivera A., Tomás M., López-Novoa J. M. Effect of adenosine A1 and A2 agonists and antagonists on cAMP and Ca2+ in cultured rat mesangial cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C840–C844. doi: 10.1152/ajpcell.1992.262.4.C840. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Fastbom J., Wiederhold K. H., Probst A. Visualization of adenosine A1 receptors in the human and the guinea-pig kidney. Eur J Pharmacol. 1987 Jun 19;138(2):273–276. doi: 10.1016/0014-2999(87)90443-2. [DOI] [PubMed] [Google Scholar]
- Sayos J., Centelles J. J., Mallol J., Canela E. I., Lluis C., Franco R. Adenosine (Ado) uptake in brush-border membrane vesicles from rat kidney (BBM). Biochem Soc Trans. 1991 Aug;19(3):323S–323S. doi: 10.1042/bst019323s. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Karlson K. H., Friedman P. A., Dietl P., Spielman W. S., Stanton B. A. Adenosine regulates a chloride channel via protein kinase C and a G protein in a rabbit cortical collecting duct cell line. J Clin Invest. 1992 Mar;89(3):834–841. doi: 10.1172/JCI115662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel N. J., Avison M. J., Reilly H. F., Alger J. R., Shulman R. G. Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol. 1983 Oct;245(4):F530–F534. doi: 10.1152/ajprenal.1983.245.4.F530. [DOI] [PubMed] [Google Scholar]
- Solsona C., Saltó C., Ymbern A. Effects of potassium depolarization on intracellular compartmentalization of ATP in cholinergic synaptosomes isolated from Torpedo electric organ. Biochim Biophys Acta. 1991 Oct 16;1095(1):57–62. doi: 10.1016/0167-4889(91)90044-x. [DOI] [PubMed] [Google Scholar]
- Spielman W. S., Arend L. J. Adenosine receptors and signaling in the kidney. Hypertension. 1991 Feb;17(2):117–130. doi: 10.1161/01.hyp.17.2.117. [DOI] [PubMed] [Google Scholar]
- Tozzi M. G., Camici M., Pesi R., Allegrini S., Sgarrella F., Ipata P. L. Nucleoside phosphotransferase activity of human colon carcinoma cytosolic 5'-nucleotidase. Arch Biochem Biophys. 1991 Dec;291(2):212–217. doi: 10.1016/0003-9861(91)90125-3. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G., Vincent M. F., Bontemps F. Pathways and control of adenine nucleotide catabolism in anoxic rat hepatocytes. Biomed Biochim Acta. 1989;48(2-3):S5–10. [PubMed] [Google Scholar]
- Weber R. G., Jones C. R., Palacios J. M., Lohse M. J. Autoradiographic visualization of A1-adenosine receptors in brain and peripheral tissues of rat and guinea pig using 125I-HPIA. Neurosci Lett. 1988 May 3;87(3):215–220. doi: 10.1016/0304-3940(88)90451-x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Humes H. D. Increases of cell ATP produced by exogenous adenine nucleotides in isolated rabbit kidney tubules. Am J Physiol. 1986 Apr;250(4 Pt 2):F720–F733. doi: 10.1152/ajprenal.1986.250.4.F720. [DOI] [PubMed] [Google Scholar]
- Worku Y., Newby A. C. Nucleoside exchange catalysed by the cytoplasmic 5'-nucleotidase. Biochem J. 1982 Sep 1;205(3):503–510. doi: 10.1042/bj2050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Churchill P. C. 2-Chloro-[3H]-adenosine binding in isolated rat kidney membranes. Arch Int Pharmacodyn Ther. 1985 Jan;273(1):83–87. [PubMed] [Google Scholar]


