Abstract
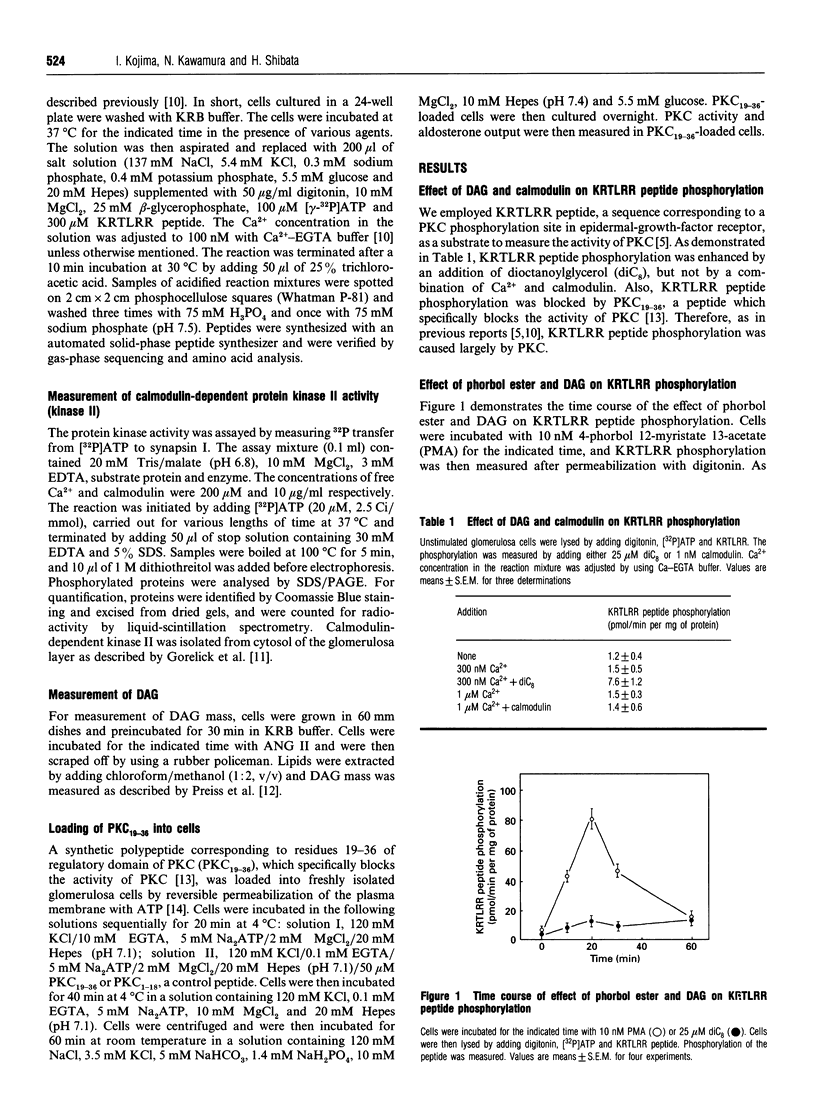
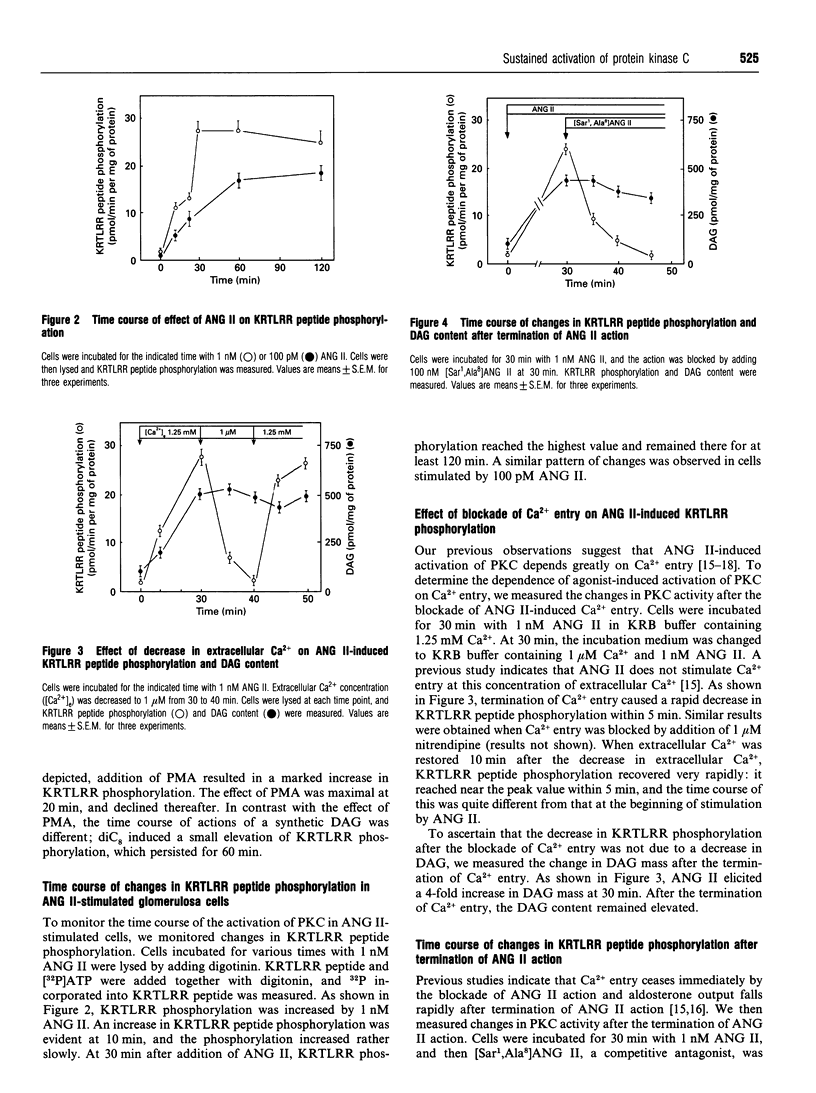
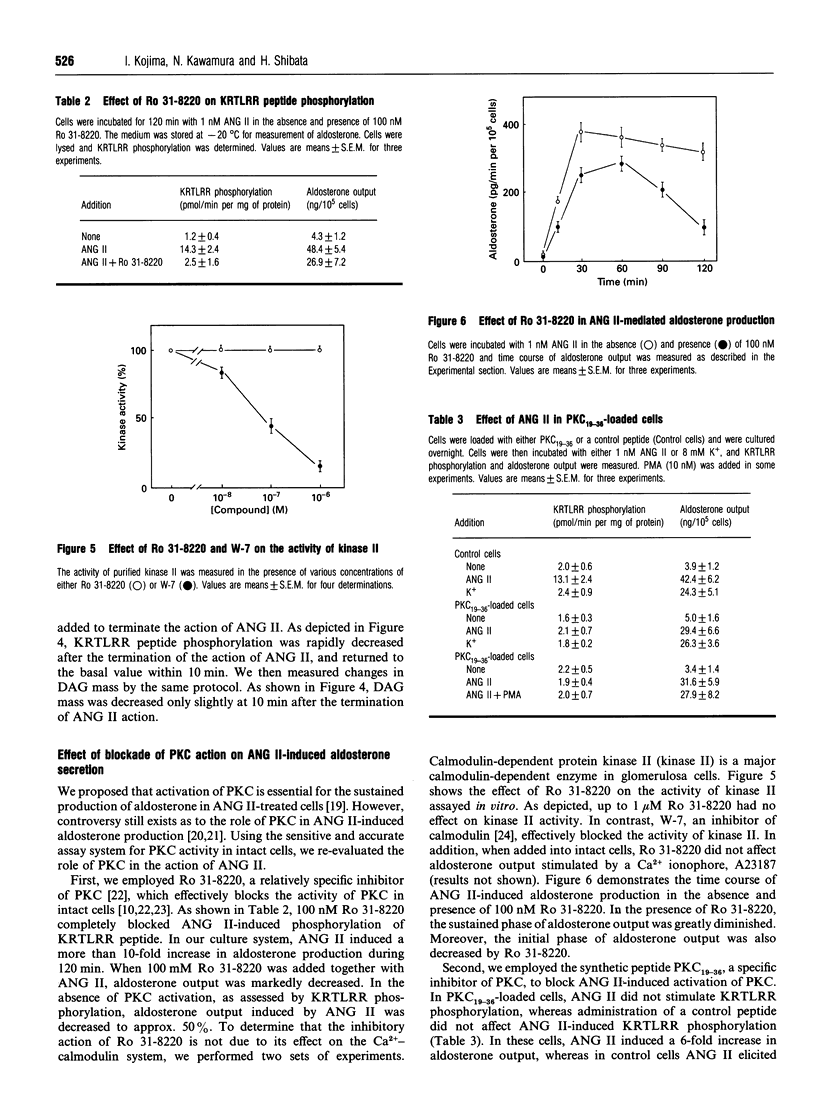
The present study was conducted to monitor precisely the activity of protein kinase C (PKC) in adrenal glomerulosa cells stimulated by angiotensin II (ANG II). PKC activity in cells was monitored by measuring phosphorylation of a synthetic KRTLRR peptide, a specific substrate for PKC, immediately after the permeabilization of the cells with digitonin [Heasley and Johnson J. Biol. Chem. (1989) 264, 8646-8652]. Addition of 1 nM ANG II induced a gradual increase in KRTLRR peptide phosphorylation, which reached a peak at 30 min, and phosphorylation was sustained thereafter. When the action of ANG II was terminated by adding [Sar1,Ala8]ANG II, a competitive antagonist, both Ca2+ entry and KRTLRR phosphorylation ceased rapidly, whereas diacylglyercol (DAG) content was not changed significantly within 10 min. Similarly, when blockade of Ca2+ entry was achieved by decreasing extracellular Ca2+ to 1 microM or by adding 1 microM nitrendipine, KRTLRR peptide phosphorylation was decreased within 5 min. In addition, restoration of Ca2+ entry was accompanied by an immediate increase in KRTLRR peptide phosphorylation. Under the same condition, DAG content did not change significantly. We then examined the role of the PKC pathway in ANG II-induced aldosterone production. Ro 31-8220 inhibited ANG II-induced KRTLRR phosphorylation without affecting the activity of calmodulin-dependent protein kinase II. In the presence of Ro 31-8220, ANG II-mediated aldosterone production was decreased to approx. 50%. Likewise, intracellular administration of PKC19-36, a sequence corresponding to residues 19-36 of the regulatory domain of PKC known to inhibit PKC activity, attenuated ANG II-mediated activation of PKC and aldosterone output. These results indicate a critical role of Ca2+ entry in the regulation of PKC activity by ANG II.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett P. Q., Bollag W. B., Isales C. M., McCarthy R. T., Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev. 1989 Nov;10(4):496–518. doi: 10.1210/edrv-10-4-496. [DOI] [PubMed] [Google Scholar]
- Barrett P. Q., Kojima I., Kojima K., Zawalich K., Isales C. M., Rasmussen H. Short term memory in the calcium messenger system. Evidence for a sustained activation of protein kinase C in adrenal glomerulosa cells. Biochem J. 1986 Sep 15;238(3):905–912. doi: 10.1042/bj2380905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. Q., Kojima I., Kojima K., Zawalich K., Isales C. M., Rasmussen H. Temporal patterns of protein phosphorylation after angiotensin II, A23187 and/or 12-O-tetradecanoylphorbol 13-acetate in adrenal glomerulosa cells. Biochem J. 1986 Sep 15;238(3):893–903. doi: 10.1042/bj2380893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Ganguly A., Chiou S., Fineberg N. S., Davis J. S. Greater importance of Ca(2+)-calmodulin in maintenance of ang II- and K(+)-mediated aldosterone secretion: lesser role of protein kinase C. Biochem Biophys Res Commun. 1992 Jan 15;182(1):254–261. doi: 10.1016/s0006-291x(05)80138-x. [DOI] [PubMed] [Google Scholar]
- Gorelick F. S., Cohn J. A., Freedman S. D., Delahunt N. G., Gershoni J. M., Jamieson J. D. Calmodulin-stimulated protein kinase activity from rat pancreas. J Cell Biol. 1983 Oct;97(4):1294–1298. doi: 10.1083/jcb.97.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Shibata H., Ogata E. Mechanism of cholinergic stimulation of aldosterone secretion in bovine adrenal glomerulosa cells. Endocrinology. 1986 Jul;119(1):284–291. doi: 10.1210/endo-119-1-284. [DOI] [PubMed] [Google Scholar]
- Kojima I., Mogami H., Shibata H., Ogata E. Role of calcium entry and protein kinase C in the progression activity of insulin-like growth factor-I in Balb/c 3T3 cells. J Biol Chem. 1993 May 15;268(14):10003–10006. [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Time-dependent restoration of the trigger pool of calcium after termination of angiotensin II action in adrenal glomerulosa cells. J Biol Chem. 1987 Apr 5;262(10):4557–4563. [PubMed] [Google Scholar]
- Nadler J. L., Natarajan R., Stern N. Specific action of the lipoxygenase pathway in mediating angiotensin II-induced aldosterone synthesis in isolated adrenal glomerulosa cells. J Clin Invest. 1987 Dec;80(6):1763–1769. doi: 10.1172/JCI113269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Carvallo P., Rocco S., Aguilera G. Role of protein kinase C on the steroidogenic effect of angiotensin II in the rat adrenal glomerulosa cell. Endocrinology. 1990 Jan;126(1):125–133. doi: 10.1210/endo-126-1-125. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Pelosin J. M., Keramidas M., Chambaz E. M. Production of platelet-activating factor is a component of the angiotensin II-protein kinase C activation pathway in bovine adrenocortical cells. Biochem J. 1991 Aug 15;278(Pt 1):29–34. doi: 10.1042/bj2780029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Sando J. J., Maurer M. C., Bolen E. J., Grisham C. M. Role of cofactors in protein kinase C activation. Cell Signal. 1992 Nov;4(6):595–609. doi: 10.1016/0898-6568(92)90041-6. [DOI] [PubMed] [Google Scholar]
- Shibata H., Kojima I. Involvement of protein kinase C in angiotensin II-mediated release of 12-hydroxyeicosatetraenoic acid in bovine adrenal glomerulosa cells. Endocrinol Jpn. 1991 Dec;38(6):611–617. doi: 10.1507/endocrj1954.38.611. [DOI] [PubMed] [Google Scholar]
- Twomey B., Muid R. E., Nixon J. S., Sedgwick A. D., Wilkinson S. E., Dale M. M. The effect of new potent selective inhibitors of protein kinase C on the neutrophil respiratory burst. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1087–1092. doi: 10.1016/0006-291x(90)90795-o. [DOI] [PubMed] [Google Scholar]
- Vinson G. P., Laird S. M., Whitehouse B. J., Hinson J. P. Specific effects of agonists of the calcium messenger system on secretion of 'late-pathway' steroid products by intact tissue and dispersed cells of the rat adrenal zona glomerulosa. J Mol Endocrinol. 1989 Mar;2(2):157–165. doi: 10.1677/jme.0.0020157. [DOI] [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]


