Abstract
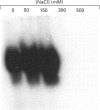
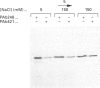
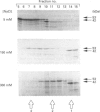
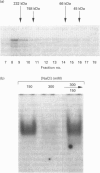
Growth suppression by p53 correlates with sequence-specific DNA binding and is determined by tertiary and quaternary protein structures. Exposure to 300 mM NaCl did not affect p53 tertiary structure, but dissociated high-molecular-mass complexes with concomitant loss of specific DNA binding. Both effects were reversible. We conclude that high salt can reversibly destabilize the quaternary structure of p53 that is most efficient for sequence-specific DNA binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook A., Milner J. Evidence for allosteric variants of wild-type p53, a tumour suppressor protein. Br J Cancer. 1990 Apr;61(4):548–552. doi: 10.1038/bjc.1990.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Bradley A. The tumor suppressor p53. Biochim Biophys Acta. 1993 Aug 23;1155(2):181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- Friedman P. N., Chen X., Bargonetti J., Prives C. The p53 protein is an unusually shaped tetramer that binds directly to DNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3319–3323. doi: 10.1073/pnas.90.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P., Hall A., Milner J. Analysis of p53 quaternary structure in relation to sequence-specific DNA binding. Oncogene. 1994 Jan;9(1):299–303. [PubMed] [Google Scholar]
- Hainaut P., Milner J. A structural role for metal ions in the "wild-type" conformation of the tumor suppressor protein p53. Cancer Res. 1993 Apr 15;53(8):1739–1742. [PubMed] [Google Scholar]
- Hainaut P., Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992 Oct;11(10):3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P., Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993 Oct 1;53(19):4469–4473. [PubMed] [Google Scholar]
- Halazonetis T. D., Davis L. J., Kandil A. N. Wild-type p53 adopts a 'mutant'-like conformation when bound to DNA. EMBO J. 1993 Mar;12(3):1021–1028. doi: 10.1002/j.1460-2075.1993.tb05743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupp T. R., Meek D. W., Midgley C. A., Lane D. P. Regulation of the specific DNA binding function of p53. Cell. 1992 Nov 27;71(5):875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- Kraiss S., Lorenz A., Montenarh M. Protein-protein interactions in high molecular weight forms of the transformation-related phosphoprotein p53. Biochim Biophys Acta. 1992 Feb 13;1119(1):11–18. doi: 10.1016/0167-4838(92)90227-5. [DOI] [PubMed] [Google Scholar]
- Medcalf E. A., Milner J. Targeting and degradation of p53 by E6 of human papillomavirus type 16 is preferential for the 1620+ p53 conformation. Oncogene. 1993 Oct;8(10):2847–2851. [PubMed] [Google Scholar]
- Meek D. W., Simon S., Kikkawa U., Eckhart W. The p53 tumour suppressor protein is phosphorylated at serine 389 by casein kinase II. EMBO J. 1990 Oct;9(10):3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne D. M., Palmer R. H., Meek D. W. Mutation of the casein kinase II phosphorylation site abolishes the anti-proliferative activity of p53. Nucleic Acids Res. 1992 Nov 11;20(21):5565–5570. doi: 10.1093/nar/20.21.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J. A conformation hypothesis for the suppressor and promoter functions of p53 in cell growth control and in cancer. Proc Biol Sci. 1991 Aug 22;245(1313):139–145. doi: 10.1098/rspb.1991.0100. [DOI] [PubMed] [Google Scholar]
- Milner J., Chan Y. S., Medcalf E. A., Wang Y., Eckhart W. Partially transformed T3T3 cells express high levels of mutant p53 in the 'wild-type' immunoreactive form with defective oligomerization. Oncogene. 1993 Jul;8(7):2001–2008. [PubMed] [Google Scholar]
- Milner J. Different forms of p53 detected by monoclonal antibodies in non-dividing and dividing lymphocytes. Nature. 1984 Jul 12;310(5973):143–145. doi: 10.1038/310143a0. [DOI] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A., Cook A. C. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991 Jan;11(1):12–19. doi: 10.1128/mcb.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991 May 31;65(5):765–774. doi: 10.1016/0092-8674(91)90384-b. [DOI] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Temperature-dependent switching between "wild-type" and "mutant" forms of p53-Val135. J Mol Biol. 1990 Dec 5;216(3):481–484. doi: 10.1016/0022-2836(90)90371-R. [DOI] [PubMed] [Google Scholar]
- Oren M. p53: the ultimate tumor suppressor gene? FASEB J. 1992 Oct;6(13):3169–3176. doi: 10.1096/fasebj.6.13.1397838. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Ha J. H., Fisher M. A. Analysis of equilibrium and kinetic measurements to determine thermodynamic origins of stability and specificity and mechanism of formation of site-specific complexes between proteins and helical DNA. Methods Enzymol. 1991;208:291–343. doi: 10.1016/0076-6879(91)08018-d. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Zauberman A., Milner J., Davies E. A., Oren M. Tight DNA binding and oligomerization are dispensable for the ability of p53 to transactivate target genes and suppress transformation. EMBO J. 1993 Jul;12(7):2789–2797. doi: 10.1002/j.1460-2075.1993.tb05940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzbecher H. W., Brain R., Addison C., Rudge K., Remm M., Grimaldi M., Keenan E., Jenkins J. R. A C-terminal alpha-helix plus basic region motif is the major structural determinant of p53 tetramerization. Oncogene. 1992 Aug;7(8):1513–1523. [PubMed] [Google Scholar]
- Tarunina M., Jenkins J. R. Human p53 binds DNA as a protein homodimer but monomeric variants retain full transcription transactivation activity. Oncogene. 1993 Nov;8(11):3165–3173. [PubMed] [Google Scholar]