Abstract
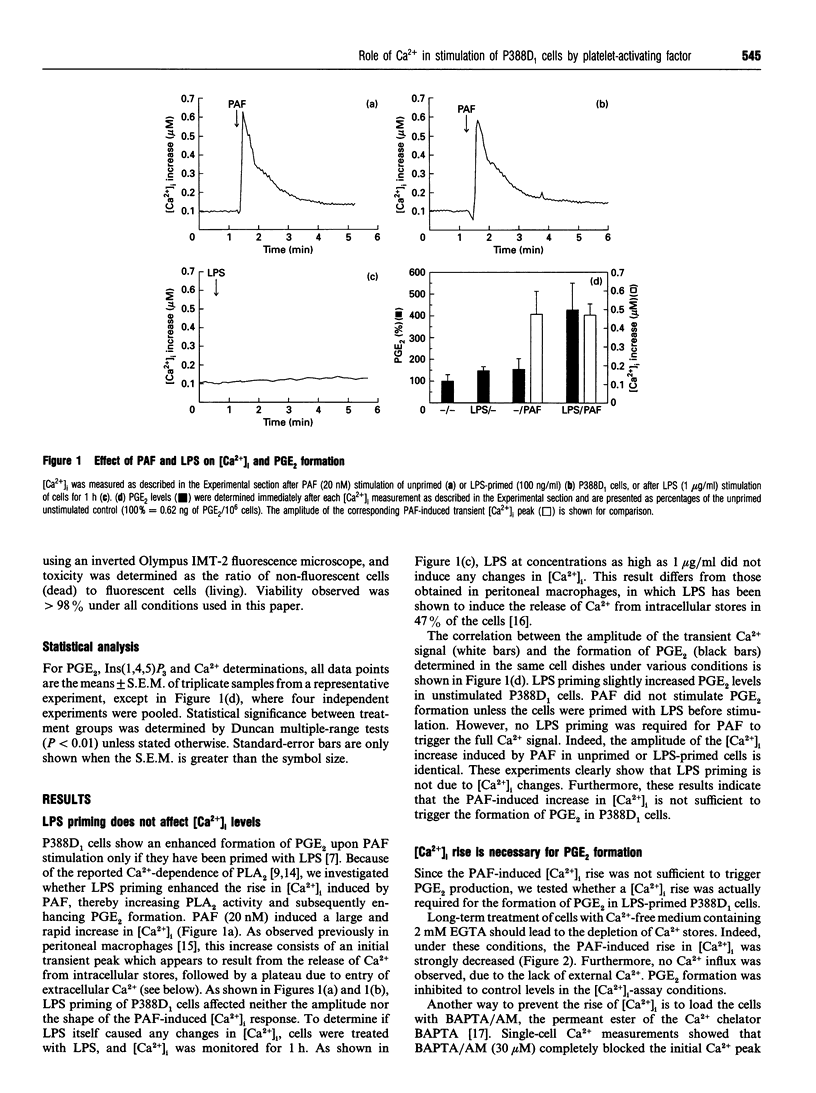
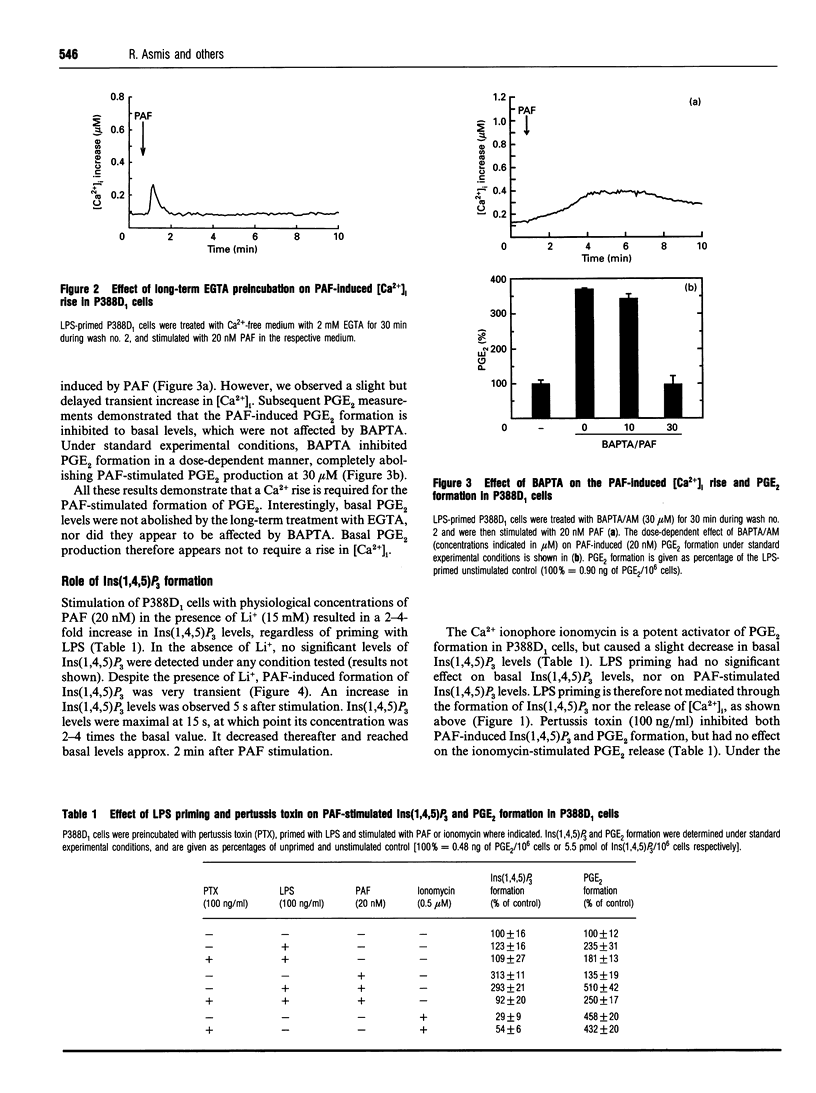
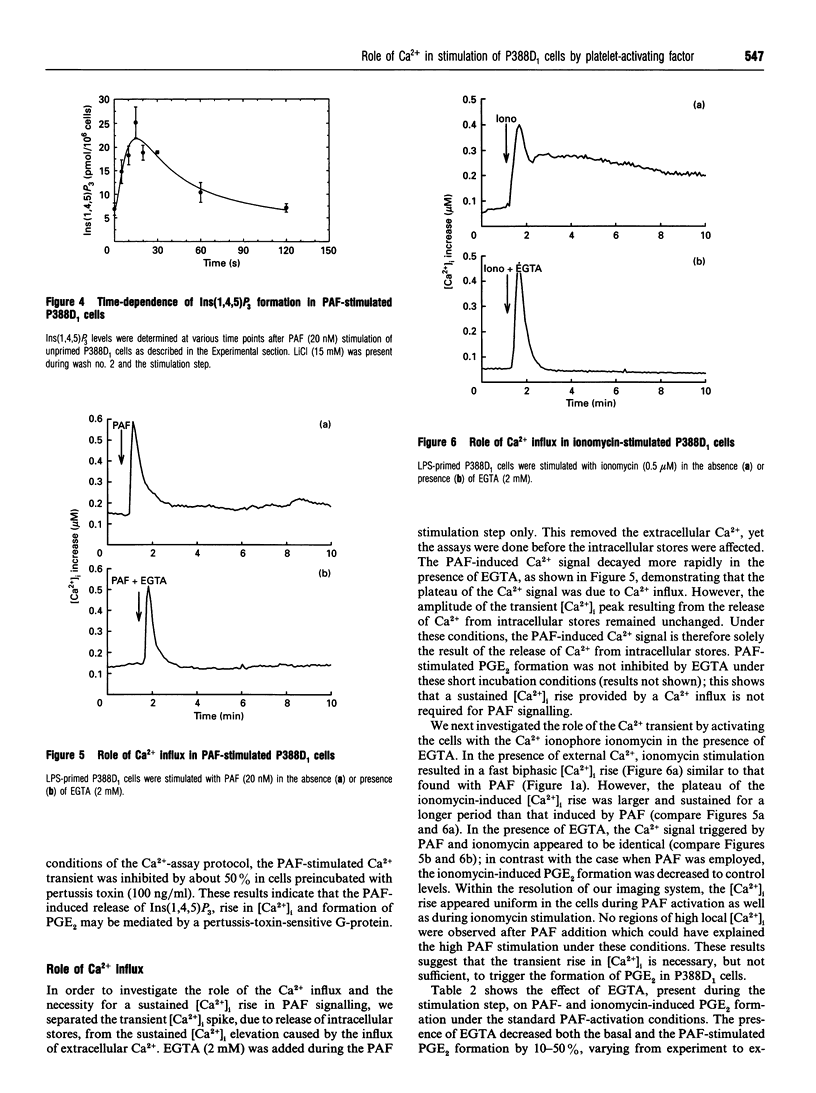
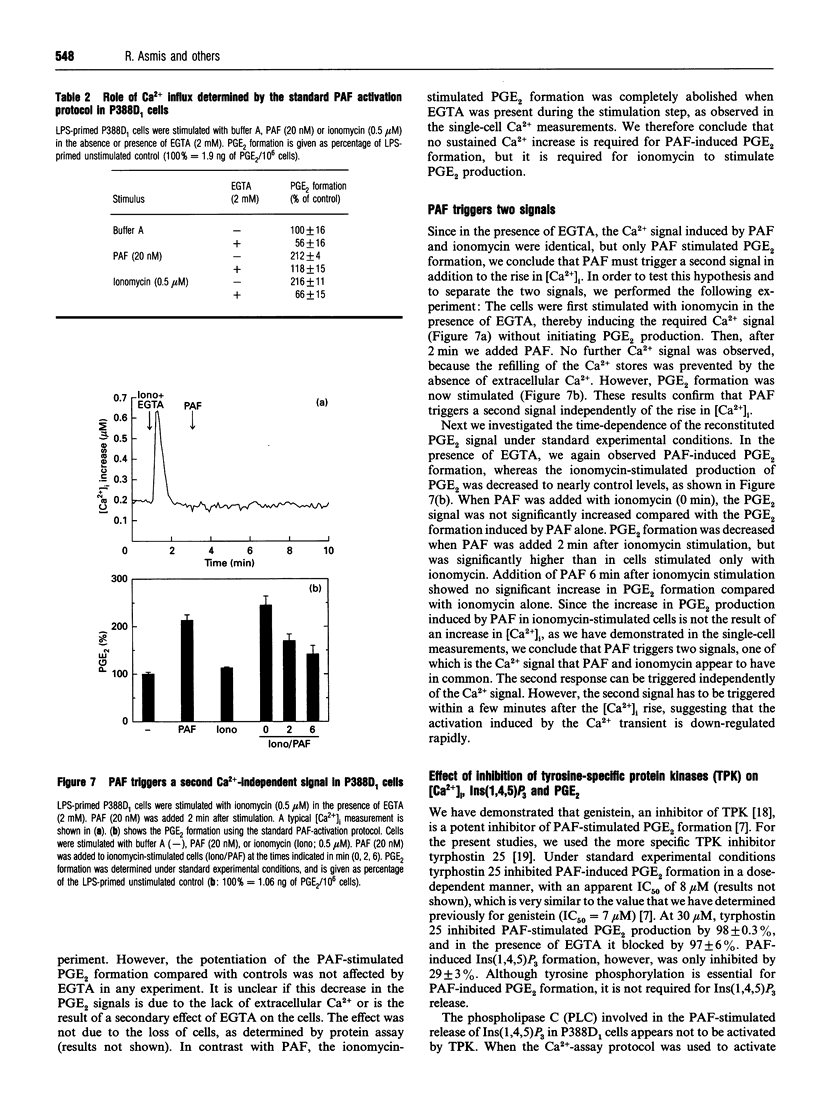
In the P388D1 macrophage-like cell line, phospholipase A2 activity and prostaglandin production are stimulated by platelet-activating factor (PAF) and bacterial lipopolysaccharide (LPS). We have investigated the role of Ins(1,4,5)P3 and Ca2+ in signal transduction of PAF-induced prostaglandin E2 (PGE2) formation in these cells. The role of Ca2+ in the activation mechanism was studied by fluorescence imaging of intracellular Ca2+ in individual adherent cells and by determining the PGE2 production in the same population of cells. This new approach enabled us to correlate directly events on the single-cell level with a physiologically relevant response of the cell population. Priming the cells with LPS was required for PAF to stimulate PGE2 formation, yet LPS affected neither the intracellular free Ca2+ concentration ([Ca2+]i) nor the PAF-induced rise in [Ca2+]i. In addition, basal and PAF-stimulated Ins(1,4,5)P3 levels were not affected by LPS priming. However, the Ca2+ transient, the release of Ins(1,4,5)P3 and the formation of PGE2 induced by PAF were inhibited in cells pretreated with pertussis toxin. Buffering the [Ca2+]i with intracellular BAPTA [bis-(o-aminophenoxy)ethane-NNN'N'-tetra-acetic acid] blocked the PAF-stimulated rise in [Ca2+]i and PGE2 formation. Removal of extracellular Ca2+ during PAF stimulation prevented the influx of Ca2+, but did not affect the initial [Ca2+]i transient, nor did it inhibit PGE2 formation. Under the same conditions, ionomycin stimulated an identical [Ca2+]i transient, but, in contrast with PAF stimulation, no PGE2 formation was observed. PGE2 production could be rescued by prompt subsequent addition of PAF, which caused no further [Ca2+]i change on its own. These results show that the transient initial rise in [Ca2+]i, produced either by PAF via the formation of Ins(1,4,5)P3 or directly by ionomycin, is necessary, but not sufficient for the formation of PGE2 in LPS-primed P388D1 cells. Furthermore, we have demonstrated for the first time that PAF triggers a second signal that is not mediated by a change in [Ca2+]i. However, both signals are required to induce PGE2 formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Barzaghi G., Sarau H. M., Mong S. Platelet-activating factor-induced phosphoinositide metabolism in differentiated U-937 cells in culture. J Pharmacol Exp Ther. 1989 Feb;248(2):559–566. [PubMed] [Google Scholar]
- Brooks R. C., McCarthy K. D., Lapetina E. G., Morell P. Receptor-stimulated phospholipase A2 activation is coupled to influx of external calcium and not to mobilization of intracellular calcium in C62B glioma cells. J Biol Chem. 1989 Nov 25;264(33):20147–20153. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Fernández B., Balsinde J. Receptor-mediated activation of arachidonic acid release in mouse peritoneal macrophages is linked to extracellular calcium influx. Biochem Biophys Res Commun. 1991 Oct 31;180(2):1036–1040. doi: 10.1016/s0006-291x(05)81170-2. [DOI] [PubMed] [Google Scholar]
- Glaser K. B., Asmis R., Dennis E. A. Bacterial lipopolysaccharide priming of P388D1 macrophage-like cells for enhanced arachidonic acid metabolism. Platelet-activating factor receptor activation and regulation of phospholipase A2. J Biol Chem. 1990 May 25;265(15):8658–8664. [PubMed] [Google Scholar]
- Huang S. J., Monk P. N., Downes C. P., Whetton A. D. Platelet-activating factor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate stimulates the production of reactive oxygen intermediates in macrophages. Biochem J. 1988 Feb 1;249(3):839–845. doi: 10.1042/bj2490839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. M., Roberts E. F., Manetta J., Putnam J. E. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem. 1991 Mar 15;266(8):5268–5272. [PubMed] [Google Scholar]
- Letari O., Nicosia S., Chiavaroli C., Vacher P., Schlegel W. Activation by bacterial lipopolysaccharide causes changes in the cytosolic free calcium concentration in single peritoneal macrophages. J Immunol. 1991 Aug 1;147(3):980–983. [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992 Nov;6(14):3275–3282. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- Lister M. D., Deems R. A., Watanabe Y., Ulevitch R. J., Dennis E. A. Kinetic analysis of the Ca2+-dependent, membrane-bound, macrophage phospholipase A2 and the effects of arachidonic acid. J Biol Chem. 1988 Jun 5;263(16):7506–7513. [PubMed] [Google Scholar]
- Lister M. D., Glaser K. B., Ulevitch R. J., Dennis E. A. Inhibition studies on the membrane-associated phospholipase A2 in vitro and prostaglandin E2 production in vivo of the macrophage-like P388D1 cell. Effects of manoalide, 7,7-dimethyl-5,8-eicosadienoic acid, and p-bromophenacyl bromide. J Biol Chem. 1989 May 25;264(15):8520–8528. [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Suganuma A., Sato M., Tohmatsu T., Nozawa Y. Mechanism of arachidonic acid liberation in platelet-activating factor-stimulated human polymorphonuclear neutrophils. J Immunol. 1989 Aug 15;143(4):1295–1302. [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. Biphasic increase in intracellular calcium induced by platelet-activating factor in macrophages. FEBS Lett. 1989 Jun 5;249(2):199–206. doi: 10.1016/0014-5793(89)80624-6. [DOI] [PubMed] [Google Scholar]
- Rhee S. G. Inositol phospholipids-specific phospholipase C: interaction of the gamma 1 isoform with tyrosine kinase. Trends Biochem Sci. 1991 Aug;16(8):297–301. doi: 10.1016/0968-0004(91)90122-c. [DOI] [PubMed] [Google Scholar]
- Ross M. I., Deems R. A., Jesaitis A. J., Dennis E. A., Ulevitch R. J. Phospholipase activities of the P388D1 macrophage-like cell line. Arch Biochem Biophys. 1985 Apr;238(1):247–258. doi: 10.1016/0003-9861(85)90162-6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Erusalimsky J., Mehmet H., Morris C., Nånberg E., Sinnett-Smith J. Signal transduction in mitogenesis: further evidence for multiple pathways. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):945–954. doi: 10.1101/sqb.1988.053.01.109. [DOI] [PubMed] [Google Scholar]
- Sharp J. D., White D. L., Chiou X. G., Goodson T., Gamboa G. C., McClure D., Burgett S., Hoskins J., Skatrud P. L., Sportsman J. R. Molecular cloning and expression of human Ca(2+)-sensitive cytosolic phospholipase A2. J Biol Chem. 1991 Aug 15;266(23):14850–14853. [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Smrcka A. V. Regulation of phospholipase C by G proteins. Trends Biochem Sci. 1992 Dec;17(12):502–506. doi: 10.1016/0968-0004(92)90340-f. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Watanabe Y., Sano M., Lister M. D., Deems R. A., Dennis E. A. Solubilization, purification, and characterization of a membrane-bound phospholipase A2 from the P388D1 macrophage-like cell line. J Biol Chem. 1988 Mar 5;263(7):3079–3085. [PubMed] [Google Scholar]


