Abstract
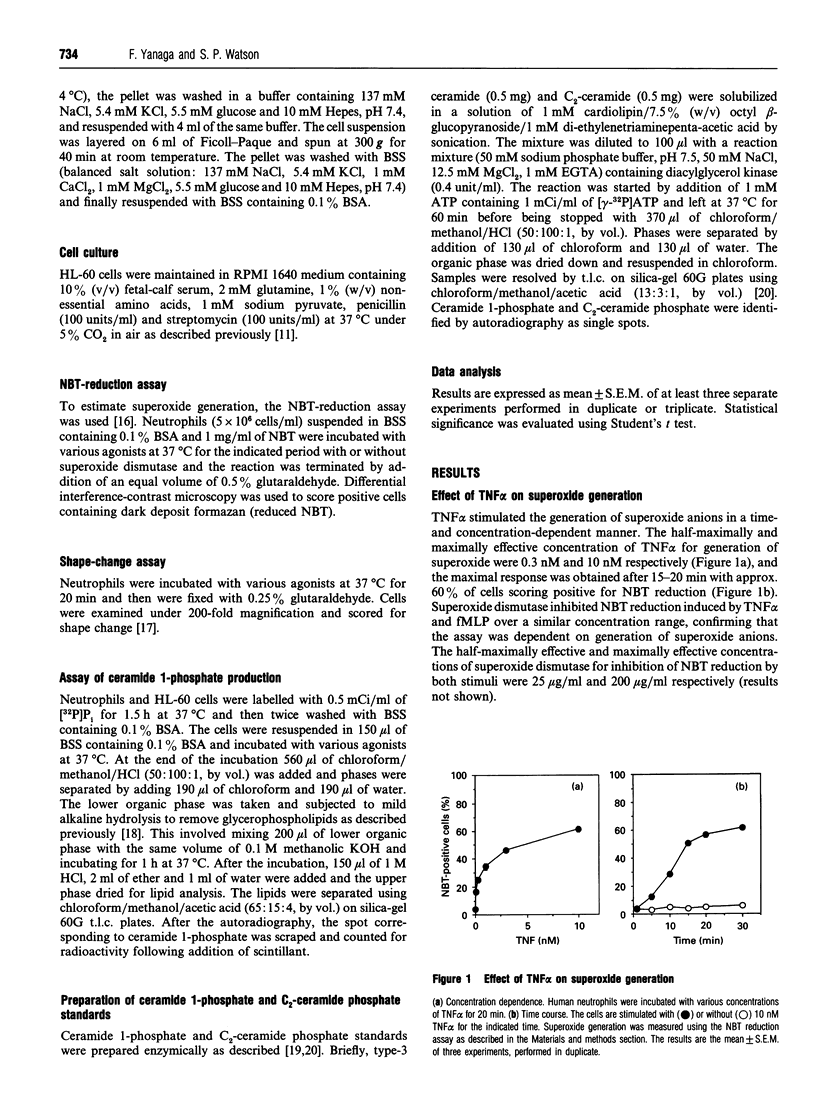
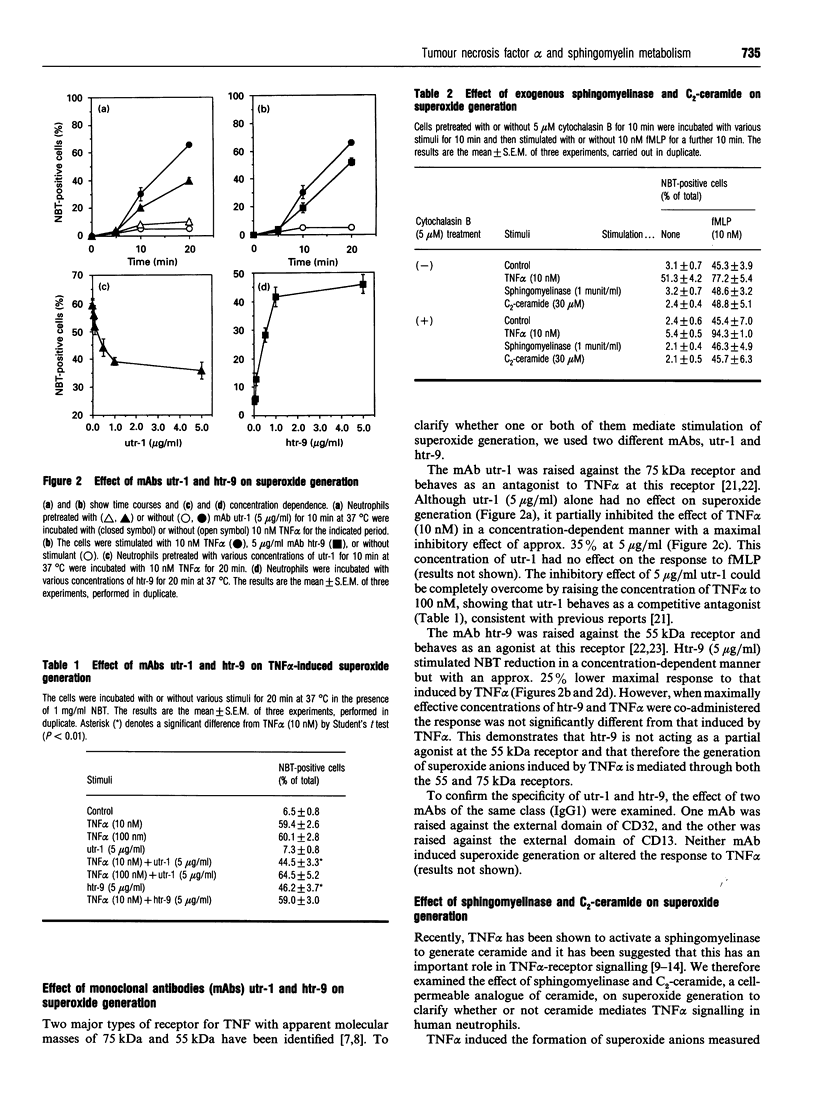
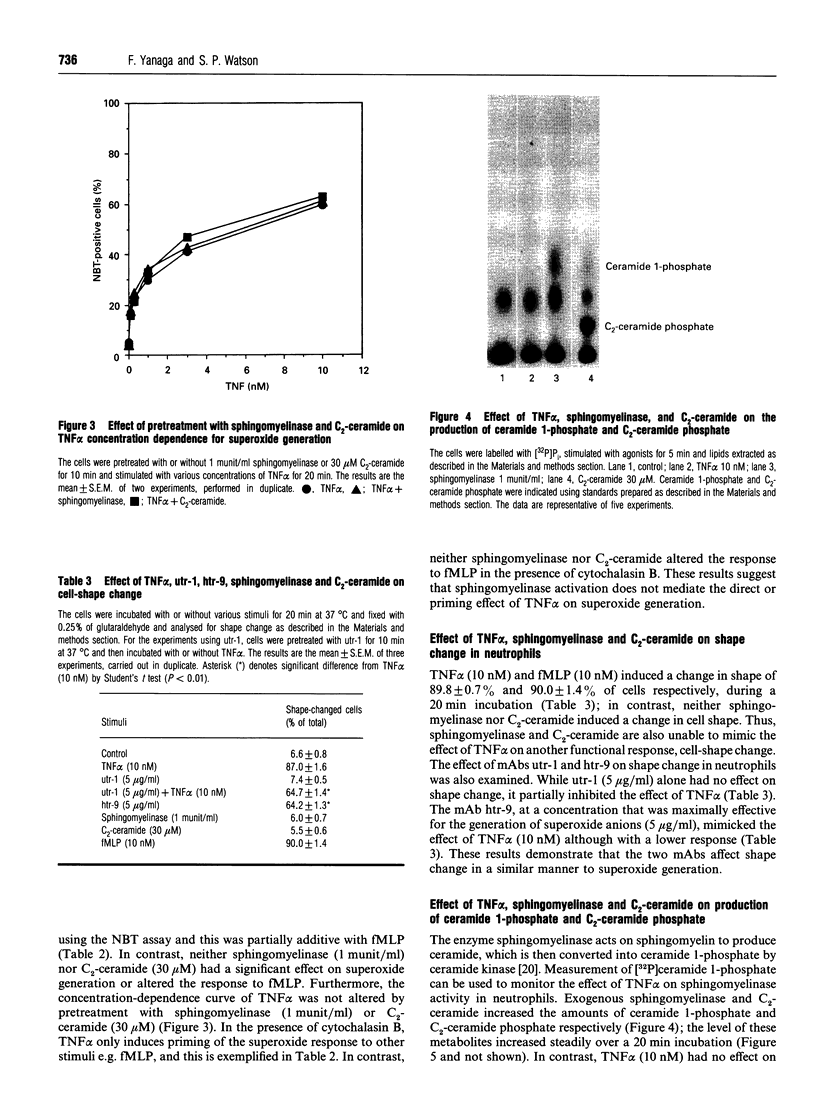
The effect of tumour necrosis factor alpha (TNF alpha) on superoxide generation in human neutrophils was investigated using the Nitro Blue Tetrazolium reduction assay. TNF alpha stimulated superoxide generation in a time- and concentration-dependent fashion. The maximally effective concentration of TNF alpha for superoxide generation was 10 nM and maximal response was obtained after 15-20 min. The monoclonal antibody (mAb), utr-1, which was raised against the 75 kDa receptor and behaves as an antagonist, had no effect on superoxide generation, but partially inhibited the response to TNF alpha. mAb htr-9, which was raised against the 55 kDa receptor and behaves as an agonist, mimicked the effect of TNF alpha, but with a lower maximal response. As it has been reported that ceramide might act as a second messenger to mediate many of the effects of TNF alpha, the effects of exogenous sphingomyelinase and the cell-permeable ceramide analogue, C2- ceramide, on production of superoxide anions, induction of priming in response to formylmethionyl-leucyl-phenylalanine, and cell-shape change were examined. Neither sphingomyelinase nor C2-ceramide mimicked the effect of TNF alpha. Ceramide is converted into ceramide 1-phosphate by ceramide kinase and we have measured levels of this metabolite to clarify the effect of TNF alpha on sphingomyelinase activity in neutrophils. Although exogenous sphingomyelinase increased the amount of ceramide 1-phosphate in a time-dependent manner, and C2-ceramide was rapidly converted into C2-ceramide phosphate, TNF alpha had no effect on the level of ceramide 1-phosphate. These results suggest that TNF alpha stimulates superoxide generation through both the 55 kDa and 75 kDa receptors, but that ceramide does not act as an intracellular mediator for TNF alpha in human neutrophils.
Full text
PDF

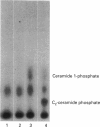
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler K. A., Kolesnick R. N. Ceramide 1-phosphate, a novel phospholipid in human leukemia (HL-60) cells. Synthesis via ceramide from sphingomyelin. J Biol Chem. 1990 Sep 5;265(25):14917–14921. [PubMed] [Google Scholar]
- Dressler K. A., Mathias S., Kolesnick R. N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992 Mar 27;255(5052):1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Holtmann H., Brakebusch C., Avni Y. S., Sarov I., Nophar Y., Hadas E., Leitner O., Wallach D. Antibodies to a soluble form of a tumor necrosis factor (TNF) receptor have TNF-like activity. J Biol Chem. 1990 Aug 25;265(24):14497–14504. [PubMed] [Google Scholar]
- Espevik T., Brockhaus M., Loetscher H., Nonstad U., Shalaby R. Characterization of binding and biological effects of monoclonal antibodies against a human tumor necrosis factor receptor. J Exp Med. 1990 Feb 1;171(2):415–426. doi: 10.1084/jem.171.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann H. P., Brockhaus M., Baeuerle P. A., Remy R., Kolbeck R., van Loon A. P. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990 Dec 25;265(36):22409–22417. [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Korchak H. M., Vosshall L. B., Zagon G., Ljubich P., Rich A. M., Weissmann G. Activation of the neutrophil by calcium-mobilizing ligands. I. A chemotactic peptide and the lectin concanavalin A stimulate superoxide anion generation but elicit different calcium movements and phosphoinositide remodeling. J Biol Chem. 1988 Aug 15;263(23):11090–11097. [PubMed] [Google Scholar]
- Kruppa G., Thoma B., Machleidt T., Wiegmann K., Krönke M. Inhibition of tumor necrosis factor (TNF)-mediated NF-kappa B activation by selective blockade of the human 55-kDa TNF receptor. J Immunol. 1992 May 15;148(10):3152–3157. [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E., Babior B. M., Curnutte J. T. Neutrophils and host defense. Ann Intern Med. 1988 Jul 15;109(2):127–142. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A., Buckley N. E., Spiegel S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1992 Dec 25;267(36):26121–26127. [PubMed] [Google Scholar]
- Park B. H., Fikrig S. M., Smithwick E. M. Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid. Lancet. 1968 Sep 7;2(7567):532–534. doi: 10.1016/s0140-6736(68)92406-9. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Kennedy E. P. Phosphorylation of ceramide by diglyceride kinase preparations from Escherichia coli. J Biol Chem. 1973 May 25;248(10):3739–3741. [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Sundan A., Loetscher H., Brockhaus M., Lesslauer W., Espevik T. Binding and regulation of cellular functions by monoclonal antibodies against human tumor necrosis factor receptors. J Exp Med. 1990 Nov 1;172(5):1517–1520. doi: 10.1084/jem.172.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J. M., Haston W. S. Behaviour of neutrophil leucocytes in uniform concentrations of chemotactic factors: contraction waves, cell polarity and persistence. J Cell Sci. 1985 Mar;74:75–93. doi: 10.1242/jcs.74.1.75. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Daniels R. H., Elmore M. A., Finnen M. J., Hill M. E., Lackie J. M. Agonist-stimulated Cl- efflux from human neutrophils. A common phenomenon during neutrophil activation. Biochem Pharmacol. 1993 May 5;45(9):1743–1751. doi: 10.1016/0006-2952(93)90429-z. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr, Goeddel D. V. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma B., Grell M., Pfizenmaier K., Scheurich P. Identification of a 60-kD tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J Exp Med. 1990 Oct 1;172(4):1019–1023. doi: 10.1084/jem.172.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Kampen E., Himmler A., Machleidt T., Krönke M. Human 55-kDa receptor for tumor necrosis factor coupled to signal transduction cascades. J Biol Chem. 1992 Sep 5;267(25):17997–18001. [PubMed] [Google Scholar]
- Yanaga F., Watson S. P. Tumor necrosis factor alpha stimulates sphingomyelinase through the 55 kDa receptor in HL-60 cells. FEBS Lett. 1992 Dec 21;314(3):297–300. doi: 10.1016/0014-5793(92)81493-6. [DOI] [PubMed] [Google Scholar]