Abstract
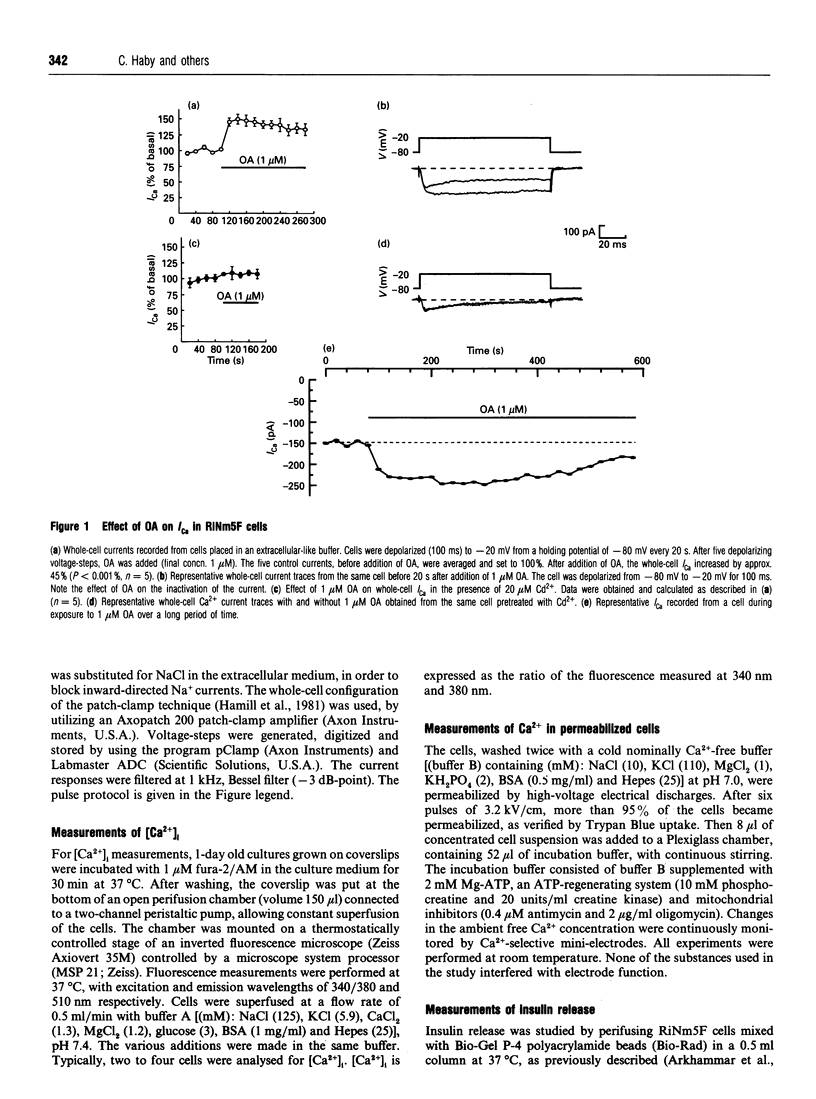
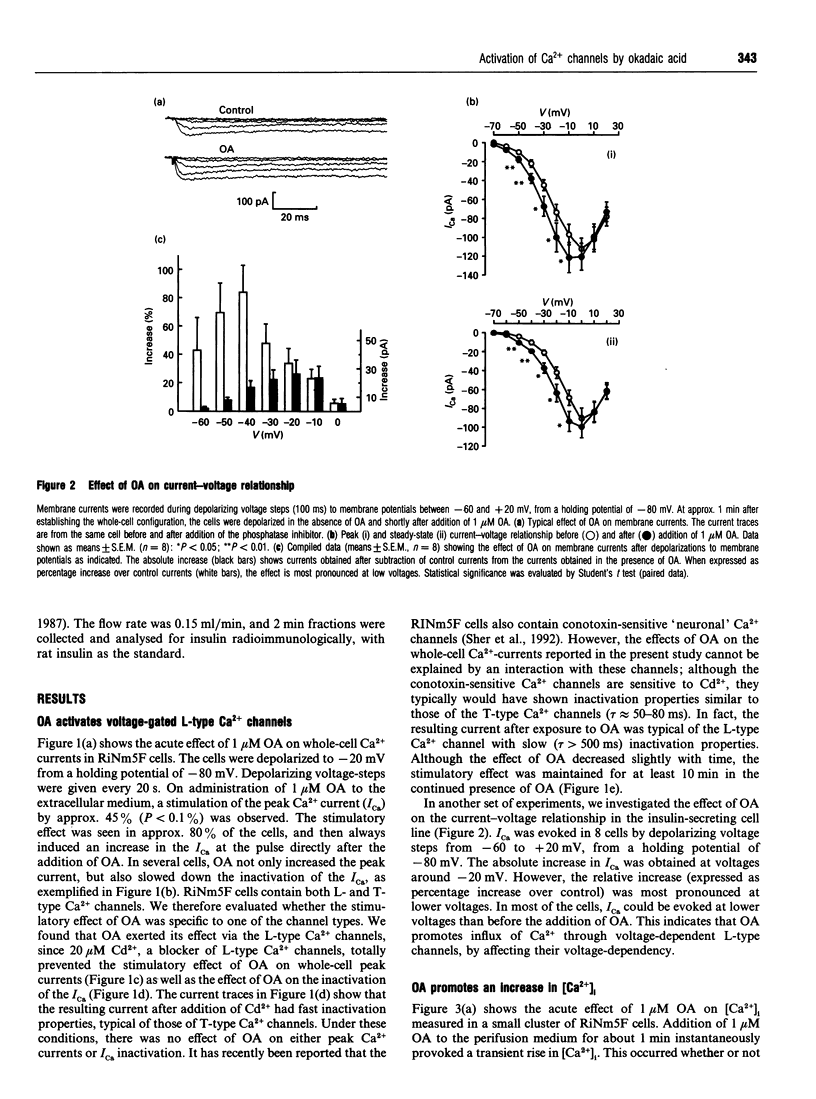
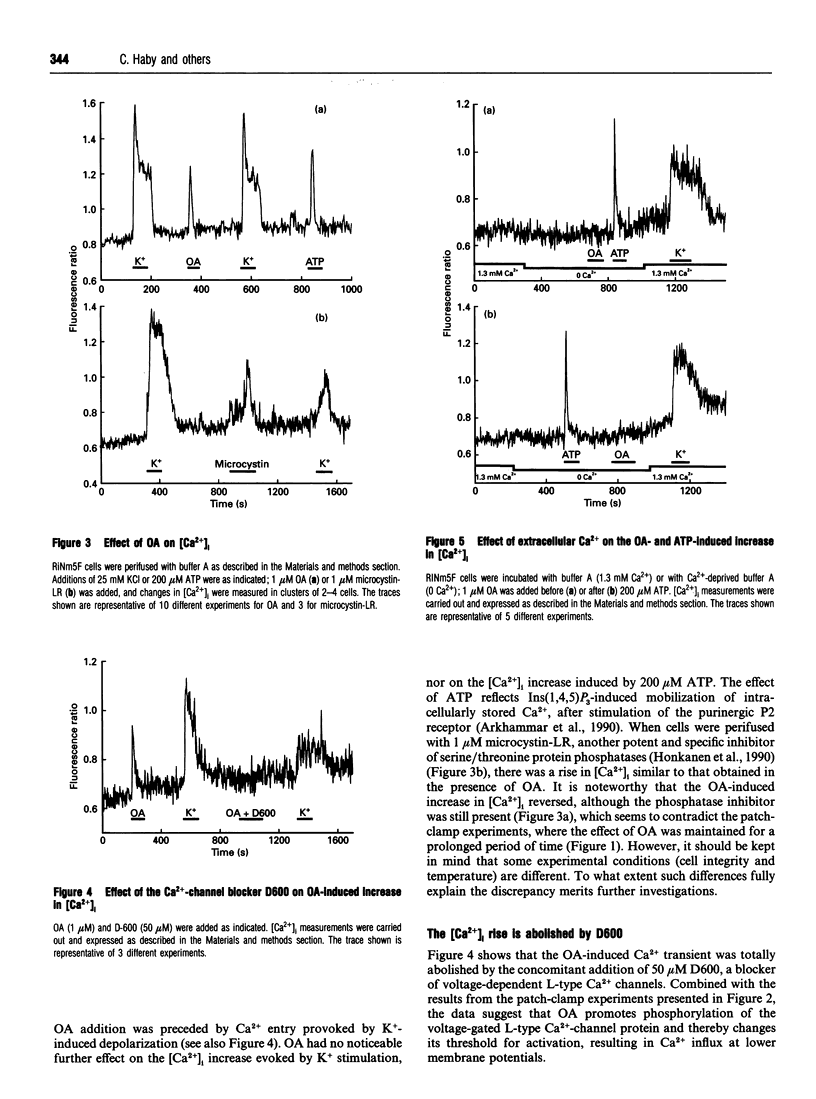
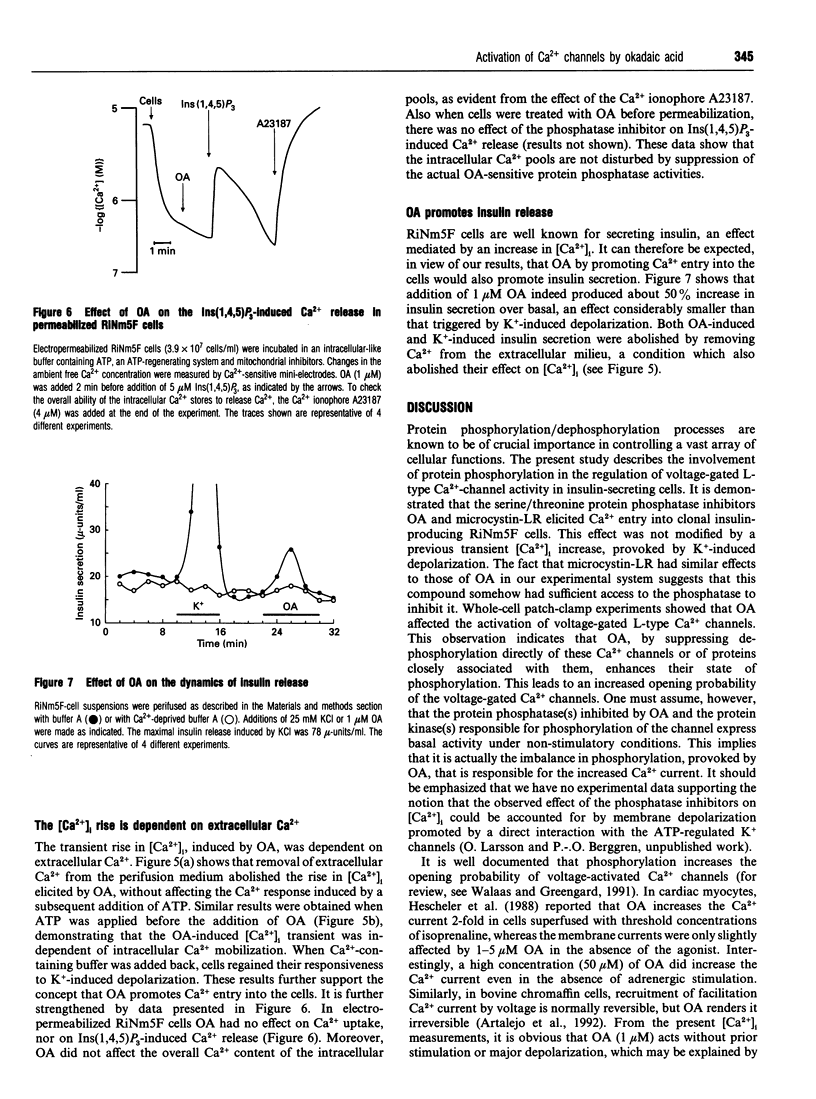
The biological activity of many proteins, including voltage-sensitive ion channels, is controlled by their state of phosphorylation. Ca2+ influx through voltage-activated L-type Ca2+ channels serves as the major stimulatory signal in insulin-secreting cells. We have now investigated the extent to which Ca2+ handling in clonal insulin-secreting RiNm5F cells was affected by okadaic acid, an inhibitor of various serine/threonine protein phosphatases. Whole-cell patch-clamp experiments showed that okadaic acid generated an increase in membrane current, suggesting that it promotes Ca2+ influx through L-type voltage-gated Ca2+ channels probably by modifying their phosphorylation state. Okadaic acid was found to provoke a transient rise in the cytoplasmic free Ca2+ concentration ([Ca2+]i) but had no further effect on the K(+)-induced increase. The Ca2+ transient induced by okadaic acid was dependent on the presence of extracellular Ca2+ and was abolished by D600, a blocker of voltage-activated L-type Ca2+ channels. Concomitant with the rise in [Ca2+]i, okadaic acid induced insulin secretion, a phenomenon that was also dependent on extracellular Ca2+. It is proposed that hyperphosphorylation of voltage-activated L-type Ca2+ channels in insulin-secreting cells lowers the threshold potential for their activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Hallberg A., Kindmark H., Nilsson T., Rorsman P., Berggren P. O. Extracellular ATP increases cytoplasmic free Ca2+ concentration in clonal insulin-producing RINm5F cells. A mechanism involving direct interaction with both release and refilling of the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. Biochem J. 1990 Jan 1;265(1):203–211. doi: 10.1042/bj2650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Rossie S., Perlman R. L., Fox A. P. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992 Jul 2;358(6381):63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Erickson A. K., Killilea S. D. Effects of fructose 2,6-bisphosphate and glucose 1,6-bisphosphate on porcine heart protein phosphatase 2A. Biochem Int. 1992 Jul;27(2):353–359. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hardie D. G., Haystead T. A., Sim A. T. Use of okadaic acid to inhibit protein phosphatases in intact cells. Methods Enzymol. 1991;201:469–476. doi: 10.1016/0076-6879(91)01042-z. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W., Mieskes G., Söling H. D. Regulation of the cardiac calcium channel by protein phosphatases. Eur J Biochem. 1987 Jun 1;165(2):261–266. doi: 10.1111/j.1432-1033.1987.tb11436.x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Honkanen R. E., Zwiller J., Daily S. L., Khatra B. S., Dukelow M., Boynton A. L. Identification, purification, and characterization of a novel serine/threonine protein phosphatase from bovine brain. J Biol Chem. 1991 Apr 5;266(10):6614–6619. [PubMed] [Google Scholar]
- Honkanen R. E., Zwiller J., Moore R. E., Daily S. L., Khatra B. S., Dukelow M., Boynton A. L. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J Biol Chem. 1990 Nov 15;265(32):19401–19404. [PubMed] [Google Scholar]
- Hosey M. M., Borsotto M., Lazdunski M. Phosphorylation and dephosphorylation of dihydropyridine-sensitive voltage-dependent Ca2+ channel in skeletal muscle membranes by cAMP- and Ca2+-dependent processes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3733–3737. doi: 10.1073/pnas.83.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D., O'Lague P. H., Erxleben C., Armstrong D. L. Calcium-dependent inactivation of the dihydropyridine-sensitive calcium channels in GH3 cells. J Gen Physiol. 1988 Oct;92(4):531–548. doi: 10.1085/jgp.92.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Biancardi E., Pollo A., Carbone E., Li G., Wollheim C. B., Clementi F. omega-Conotoxin-sensitive, voltage-operated Ca2+ channels in insulin-secreting cells. Eur J Pharmacol. 1992 Jun 17;216(3):407–414. doi: 10.1016/0014-2999(92)90438-a. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Vu N. D. Regulation of norepinephrine secretion in permeabilized PC12 cells by Ca2(+)-stimulated phosphorylation. Effects of protein phosphatases and phosphatase inhibitors. J Biol Chem. 1990 Jun 25;265(18):10352–10357. [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Wu Y. N., Wagner P. D. Effects of phosphatase inhibitors and a protein phosphatase on norepinephrine secretion by permeabilized bovine chromaffin cells. Biochim Biophys Acta. 1991 May 17;1092(3):384–390. doi: 10.1016/s0167-4889(97)90016-1. [DOI] [PubMed] [Google Scholar]
- Yanagihara N., Toyohira Y., Koda Y., Wada A., Izumi F. Inhibitory effect of okadaic acid on carbachol-evoked secretion of catecholamines in cultured bovine adrenal medullary cells. Biochem Biophys Res Commun. 1991 Jan 15;174(1):77–83. doi: 10.1016/0006-291x(91)90487-r. [DOI] [PubMed] [Google Scholar]


