Abstract
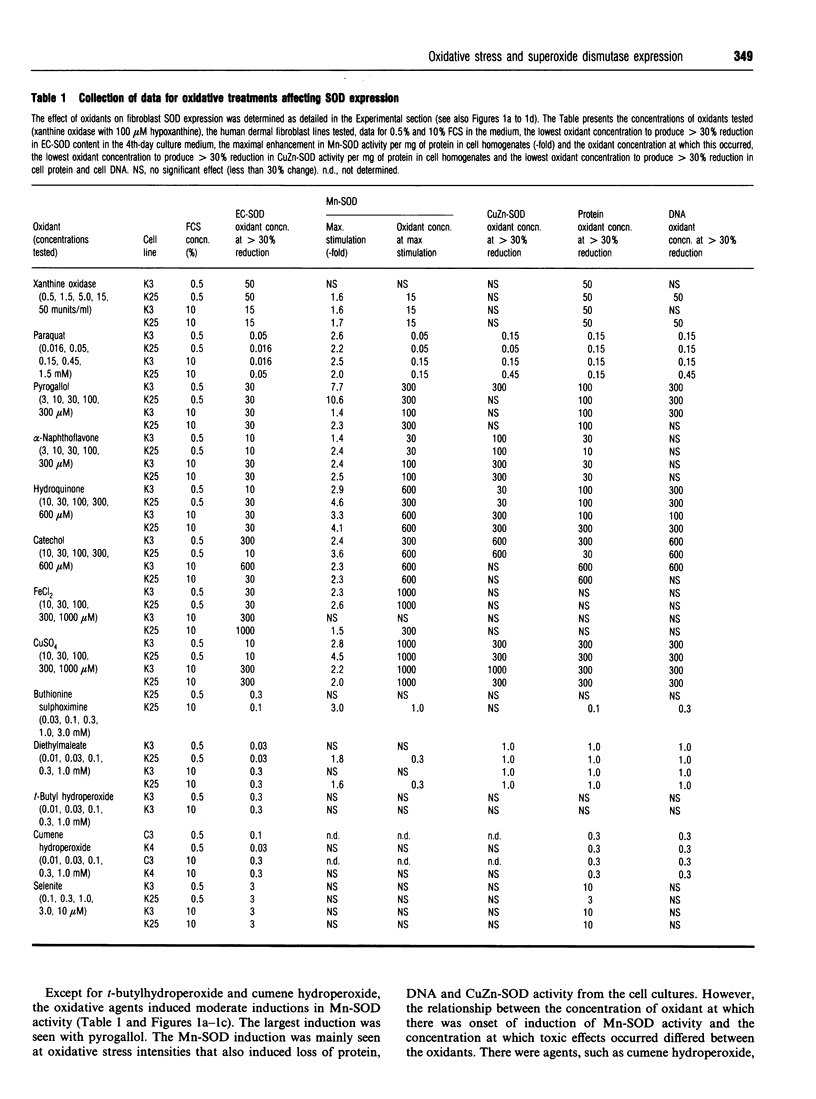
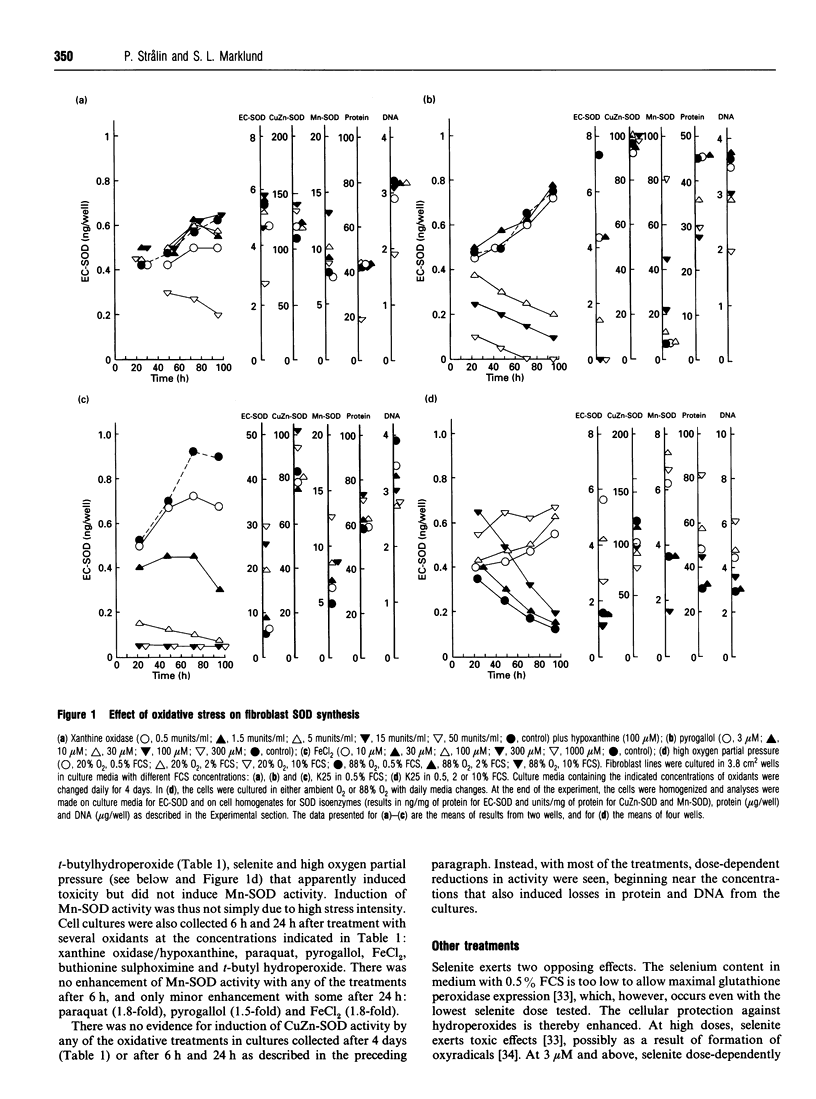
To determine the effect of oxidative stress on expression of extracellular superoxide dismutase (EC-SOD), CuZn-SOD and Mn-SOD, two fibroblast lines were exposed for periods of up to 4 days to a wide concentration range of oxidizing agents: xanthine oxidase plus hypoxanthine, paraquat, pyrogallol, alpha-naphthoflavone, hydroquinone, catechol, Fe2+ ions, Cu2+ ions, buthionine sulphoximine, diethylmaleate, t-butyl hydroperoxide, cumene hydroperoxide, selenite, citiolone and high oxygen partial pressure. The cell lines were cultured both under serum starvation and at a serum concentration that permitted growth. Under no condition was there any evidence of EC-SOD induction. Instead, the agents uniformly, dose-dependently and continuously reduced EC-SOD expression. We interpret the effect to be due to toxicity. Enhancement of the protection against oxidative stress by addition of CuZn-SOD, catalase and low concentrations of selenite did not influence the expression of any of the SOD isoenzymes. Removal of EC-SOD from cell surfaces by heparin also did not influence SOD expression. Mn-SOD was moderately induced by high doses of the first 11 oxidants. Apart from reduction at high toxic doses, there were no significant effects on the CuZn-SOD activity by any of the treatments. Thus EC-SOD, previously shown to be profoundly influenced by inflammatory cytokines, was not induced by its substrate or other oxidants. In a similar fashion, Mn-SOD, previously shown to be greatly induced and depressed by cytokines, was only moderately influenced by oxidants. We suggest that the regulation of these SOD isoenzymes in mammalian tissues primarily occurs in a manner co-ordinated by cytokines, rather than as a response of individual cells to oxidants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala Y., Palluy O., Favero J., Bonne C., Modat G., Dornand J. Hypoxia/reoxygenation stimulates endothelial cells to promote interleukin-1 and interleukin-6 production. Effects of free radical scavengers. Agents Actions. 1992 Sep;37(1-2):134–139. doi: 10.1007/BF01987902. [DOI] [PubMed] [Google Scholar]
- Allen R. G. Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med. 1991 Feb;196(2):117–129. doi: 10.3181/00379727-196-43171a. [DOI] [PubMed] [Google Scholar]
- Auwerx J. H., Chait A., Wolfbauer G., Deeb S. S. Loss of copper-zinc superoxide dismutase gene expression in differentiated cells of myelo-monocytic origin. Blood. 1989 Oct;74(5):1807–1810. [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bump E. A., Yu N. Y., Brown J. M. Radiosensitization of hypoxic tumor cells by depletion of intracellular glutathione. Science. 1982 Aug 6;217(4559):544–545. doi: 10.1126/science.7089580. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clerch L. B., Massaro D. Rat lung antioxidant enzymes: differences in perinatal gene expression and regulation. Am J Physiol. 1992 Oct;263(4 Pt 1):L466–L470. doi: 10.1152/ajplung.1992.263.4.L466. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M. Oxygen-induced changes in pulmonary superoxide dismutase assayed by antibody titrations. Am J Physiol. 1976 Oct;231(4):1196–1203. doi: 10.1152/ajplegacy.1976.231.4.1196. [DOI] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Donati Y. R., Slosman D. O., Polla B. S. Oxidative injury and the heat shock response. Biochem Pharmacol. 1990 Dec 15;40(12):2571–2577. doi: 10.1016/0006-2952(90)90573-4. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fisher A. B. Antioxidant enzymes of rat granular pneumocytes. Constitutive levels and effect of hyperoxia. Lab Invest. 1981 Jul;45(1):1–6. [PubMed] [Google Scholar]
- Frank L. Developmental aspects of experimental pulmonary oxygen toxicity. Free Radic Biol Med. 1991;11(5):463–494. doi: 10.1016/0891-5849(91)90062-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gage J. C. The action of paraquat and diquat on the respiration of liver cell fractions. Biochem J. 1968 Oct;109(5):757–761. doi: 10.1042/bj1090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougerot-Pocidalo M. A., Roche Y., Fay M., Perianin A., Bailly S. Oxidative injury amplifies interleukin-1-like activity produced by human monocytes. Int J Immunopharmacol. 1989;11(8):961–969. doi: 10.1016/0192-0561(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass M. A., Massaro D. Regulation of the synthesis of superoxide dismutases in rat lungs during oxidant and hyperthermic stresses. J Biol Chem. 1988 Jan 15;263(2):776–781. [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Howard A. J., Crapo J. D. Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am J Respir Cell Mol Biol. 1991 Mar;4(3):278–286. doi: 10.1165/ajrcmb/4.3.278. [DOI] [PubMed] [Google Scholar]
- Housset B., Junod A. F. Effects of culture conditions and hyperoxia on antioxidant enzymes in pig pulmonary artery and aortic endothelium. Biochim Biophys Acta. 1982 Jun 16;716(3):283–289. doi: 10.1016/0304-4165(82)90018-6. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Clerch L. B., Hass M. A., Frank L., Massaro D. Endotoxin increases lung Cu,Zn superoxide dismutase mRNA: O2 raises enzyme synthesis. Am J Physiol. 1989 Aug;257(2 Pt 1):L61–L64. doi: 10.1152/ajplung.1989.257.2.L61. [DOI] [PubMed] [Google Scholar]
- Jensen J. C., Pogrebniak H. W., Pass H. I., Buresh C., Merino M. J., Kauffman D., Venzon D., Langstein H. N., Norton J. A. Role of tumor necrosis factor in oxygen toxicity. J Appl Physiol (1985) 1992 May;72(5):1902–1907. doi: 10.1152/jappl.1992.72.5.1902. [DOI] [PubMed] [Google Scholar]
- Jornot L., Junod A. F. Response of human endothelial cell antioxidant enzymes to hyperoxia. Am J Respir Cell Mol Biol. 1992 Jan;6(1):107–115. doi: 10.1165/ajrcmb/6.1.107. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Lindahl U., Marklund S. L. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem J. 1988 Nov 15;256(1):29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Binding of human extracellular-superoxide dismutase C to cultured cell lines and to blood cells. Lab Invest. 1989 May;60(5):659–666. [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988 Oct 1;255(1):223–228. [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Plasma clearance of human extracellular-superoxide dismutase C in rabbits. J Clin Invest. 1988 Sep;82(3):762–766. doi: 10.1172/JCI113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X. J., Fanburg B. L. Regulation of Cu,Zn-superoxide dismutase in bovine pulmonary artery endothelial cells. J Cell Physiol. 1992 Dec;153(3):491–497. doi: 10.1002/jcp.1041530308. [DOI] [PubMed] [Google Scholar]
- Kumar S., Björnstedt M., Holmgren A. Selenite is a substrate for calf thymus thioredoxin reductase and thioredoxin and elicits a large non-stoichiometric oxidation of NADPH in the presence of oxygen. Eur J Biochem. 1992 Jul 15;207(2):435–439. doi: 10.1111/j.1432-1033.1992.tb17068.x. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990 Feb 15;266(1):213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992 Apr 5;267(10):6696–6701. [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Masuda A., Longo D. L., Kobayashi Y., Appella E., Oppenheim J. J., Matsushima K. Induction of mitochondrial manganese superoxide dismutase by interleukin 1. FASEB J. 1988 Dec;2(15):3087–3091. doi: 10.1096/fasebj.2.15.3263930. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meltzer S. J., Mane S. M., Wood P. K., Johnson L., Needleman S. W. Sequencing products of the polymerase chain reaction directly, without purification. Biotechniques. 1990 Feb;8(2):142–148. [PubMed] [Google Scholar]
- Nicotera T. M., Block A. W., Gibas Z., Sandberg A. A. Induction of superoxide dismutase, chromosomal aberrations and sister-chromatid exchanges by paraquat in Chinese hamster fibroblasts. Mutat Res. 1985 Sep;151(2):263–268. doi: 10.1016/0027-5107(85)90078-8. [DOI] [PubMed] [Google Scholar]
- Papaccio G., Pisanti F. A., Frascatore S. Acetyl-homocysteine-thiolactone-induced increase of superoxide dismutase counteracts the effect of subdiabetogenic doses of streptozocin. Diabetes. 1986 Apr;35(4):470–474. doi: 10.2337/diab.35.4.470. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 1976 Nov;58(5):1174–1184. doi: 10.1172/JCI108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R., Pickett C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991 Jun 25;266(18):11632–11639. [PubMed] [Google Scholar]
- Sandström B. E., Carlsson J., Marklund S. L. Variations among cultured cells in glutathione peroxidase activity in response to selenite supplementation. Biochim Biophys Acta. 1987 Jul 6;929(2):148–153. doi: 10.1016/0167-4889(87)90170-4. [DOI] [PubMed] [Google Scholar]
- Sandström J., Karlsson K., Edlund T., Marklund S. L. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J. 1993 Sep 15;294(Pt 3):853–857. doi: 10.1042/bj2940853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull S., Heintz N. H., Periasamy M., Manohar M., Janssen Y. M., Marsh J. P., Mossman B. T. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991 Dec 25;266(36):24398–24403. [PubMed] [Google Scholar]
- Steinkühler C., Sapora O., Carrì M. T., Nagel W., Marcocci L., Ciriolo M. R., Weser U., Rotilio G. Increase of Cu,Zn-superoxide dismutase activity during differentiation of human K562 cells involves activation by copper of a constantly expressed copper-deficient protein. J Biol Chem. 1991 Dec 25;266(36):24580–24587. [PubMed] [Google Scholar]
- Stevens J. B., Autor A. P. Induction of superoxide dismutase by oxygen in neonatal rat lung. J Biol Chem. 1977 May 25;252(10):3509–3514. [PubMed] [Google Scholar]
- Stevens J. B., Autor A. P. Oxygen-induced synthesis of superoxide dismutase and catalase in pulmonary macrophages of neonatal rats. Lab Invest. 1977 Nov;37(5):470–478. [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda T., Nomura I., Kurashige T., Kubonishi I., Miyoshi I., Sukenaga Y., Taniguchi T. Changes in Cu,Zn-superoxide dismutase gene during induced erythroid and myeloid differentiation. Acta Haematol. 1991;86(4):183–188. doi: 10.1159/000204831. [DOI] [PubMed] [Google Scholar]
- Westerbeek-Marres C. A., Moore M. M., Autor A. P. Regulation of manganese superoxide dismutase in Saccharomyces cerevisiae. The role of respiratory chain activity. Eur J Biochem. 1988 Jul 1;174(4):611–620. doi: 10.1111/j.1432-1033.1988.tb14142.x. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]


