Abstract
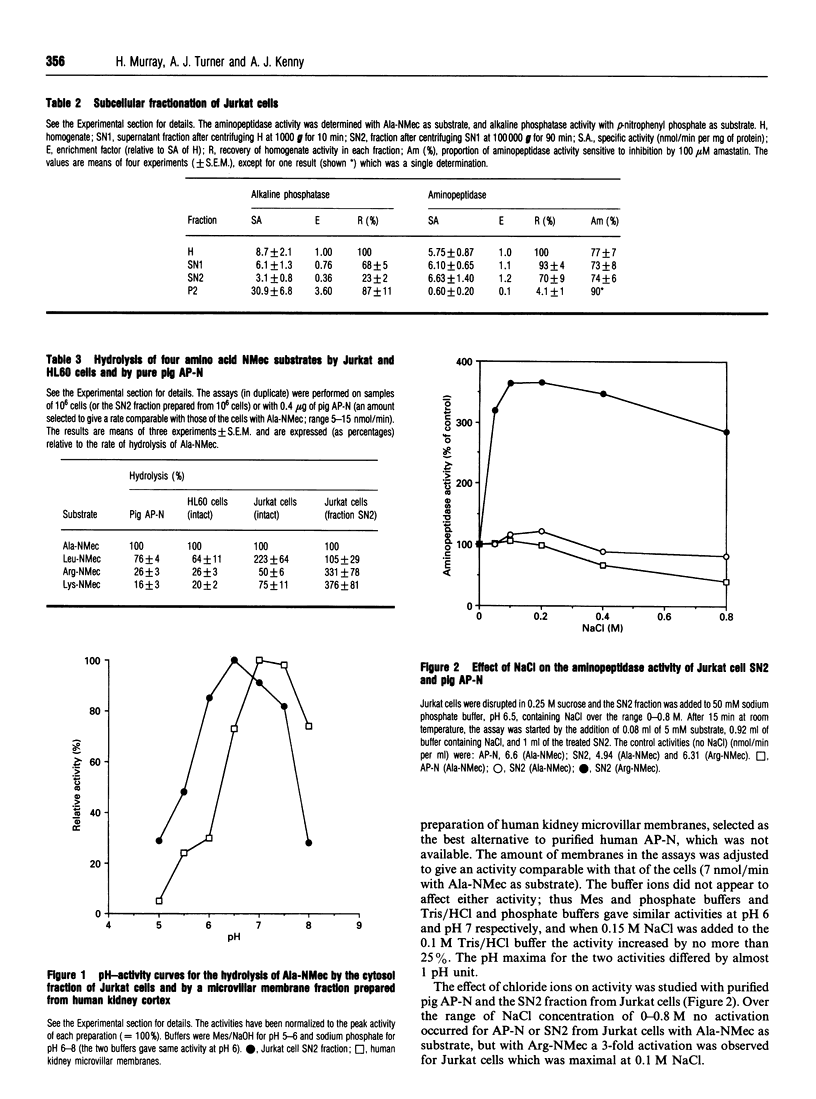
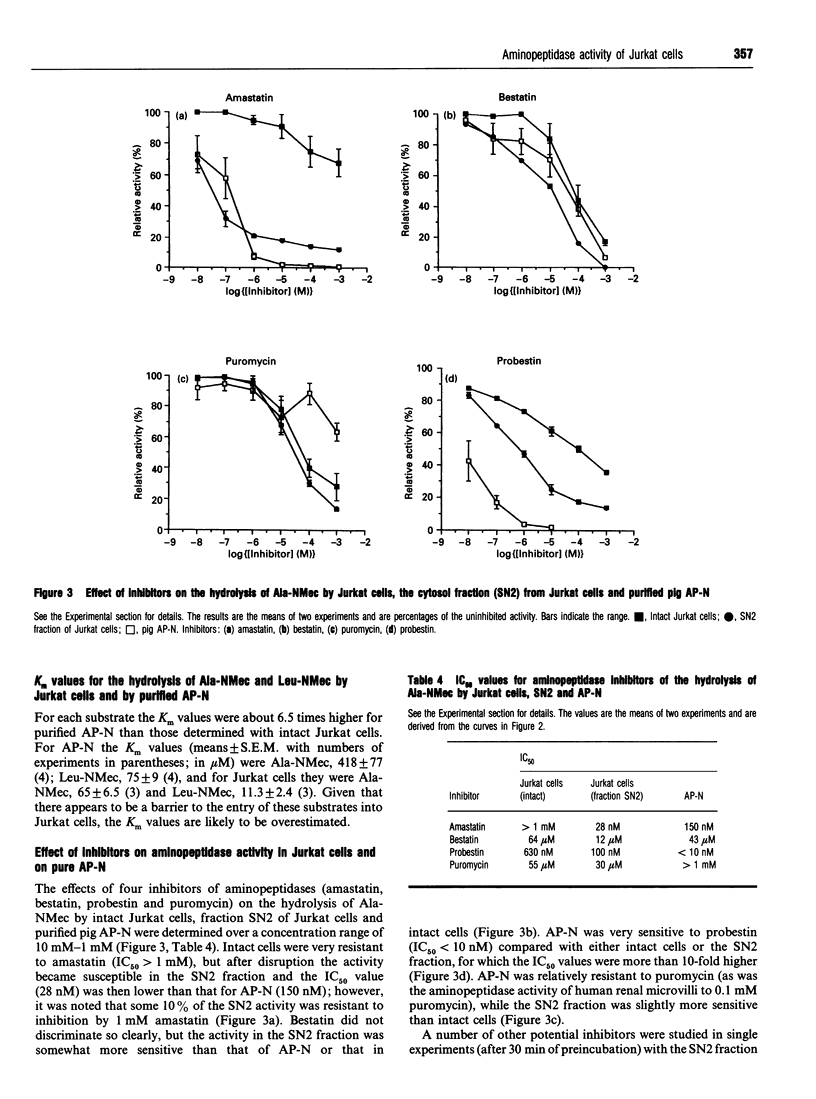
Although lymphocytes are CD-13-negative and therefore should not express the ectoenzyme aminopeptidase N (AP-N), there have been a number of reports suggesting the presence of a cell-surface aminopeptidase with many similarities to AP-N. We have determined aminopeptidase activity with 4-methyl-7-coumarylamide (NMec) derivatives of alanine, leucine, lysine and arginine in Jurkat cells (a human T-cell lymphoma line) and in HL60 cells (a CD-13-positive myeloid leukaemia line) and compared the activities with those of purified pig AP-N and human renal microvillar membranes. Jurkat cell aminopeptidase activity doubled on disrupting the cells and the sensitivity to amastatin increased. When the cells were fractionated only 4% of the activity was recovered in the membrane fraction, compared with 87% recovery for alkaline phosphatase. The profile of activities for intact Jurkat cells was Leu > Ala > Lys > Arg, changing in the cytosolic fraction to Lys > or = Arg > Leu = Ala; the profiles for intact HL60 cells and AP-N were identical, namely Ala > Leu > Arg > Lys. The Km values for the hydrolysis of Ala-NMec and Leu-NMec by Jurkat cells were 65 microM and 11 microM, in each case some 6-fold lower than those for AP-N. The pH-activity curves for the hydrolysis of Ala-NMec by Jurkat cells and human renal microvillar membranes were displaced by almost 1 pH unit and the activity was not sensitive to the anionic composition of the buffers. However, a 3-fold activation of the cytosolic activity by 0.1 M NaCl was observed with Arg-NMec as substrate. With Ala-NMec as substrate, the sensitivity of the aminopeptidase activity to inhibitors increased markedly after disrupting the cells, but still differed from that observed with purified pig AP-N; the concentrations giving 50% inhibition were as follows (values for AP-N in parentheses): amastatin. 28 nM (150 nM); bestatin, 12 microM (43 microM), probestin, 100 nM (< 10 nM), puromycin, 30 microM (> 1 mM). Anion exchange chromatography on Mono Q revealed two activities: that of peak I preferentially hydrolysed Arg-NMec, was activated by NaCl and was insensitive to amastatin; while that of peak II was strongly inhibited by amastatin and had a broad specificity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramić M., Vitale L. Basic amino acids preferring broad specificity aminopeptidase from human erythrocytes. Biol Chem Hoppe Seyler. 1992 Jul;373(7):375–380. doi: 10.1515/bchm3.1992.373.2.375. [DOI] [PubMed] [Google Scholar]
- Amoscato A. A., Alexander J. W., Babcock G. F. Surface aminopeptidase activity of human lymphocytes. I. Biochemical and biologic properties of intact cells. J Immunol. 1989 Feb 15;142(4):1245–1252. [PubMed] [Google Scholar]
- Amoscato A. A., Balasubramaniam A., Alexander J. W., Babcock G. F. Degradation of thymopentin by human lymphocytes: evidence for aminopeptidase activity. Biochim Biophys Acta. 1988 Jul 20;955(2):164–174. doi: 10.1016/0167-4838(88)90190-2. [DOI] [PubMed] [Google Scholar]
- Amoscato A. A., Sramkoski R. M., Babcock G. F., Alexander J. W. Neutral surface aminopeptidase activity of human tumor cell lines. Biochim Biophys Acta. 1990 Dec 5;1041(3):317–319. doi: 10.1016/0167-4838(90)90291-m. [DOI] [PubMed] [Google Scholar]
- Ansorge S., Schön E., Kunz D. Membrane-bound peptidases of lymphocytes: functional implications. Biomed Biochim Acta. 1991;50(4-6):799–807. [PubMed] [Google Scholar]
- Ashmun R. A., Look A. T. Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood. 1990 Jan 15;75(2):462–469. [PubMed] [Google Scholar]
- Barnes K., Ingram J., Kenny A. J. Proteins of the kidney microvillar membrane. Structural and immunochemical properties of rat endopeptidase-2 and its immunohistochemical localization in tissues of rat and mouse. Biochem J. 1989 Dec 1;264(2):335–346. doi: 10.1042/bj2640335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B. Murine thymocytes possess specific cell surface-associated exoaminopeptidase activities: preferential expression by immature CD4-CD8- subpopulation. Eur J Immunol. 1990 Mar;20(3):459–468. doi: 10.1002/eji.1830200302. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes M. A., Kenny A. J. An immunohistochemical study of endopeptidase-24.11 and aminopeptidase N in lymphoid tissues. Immunology. 1987 Feb;60(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Evans J. F., Kargman S. Bestatin inhibits covalent coupling of [3H]LTA4 to human leukocyte LTA4 hydrolase. FEBS Lett. 1992 Feb 3;297(1-2):139–142. doi: 10.1016/0014-5793(92)80345-h. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Kenny A. J. Proteins of the kidney microvillar membrane. The 130 kDa protein in pig kidney, recognized by monoclonal antibody GK5C1, is an ectoenzyme with aminopeptidase activity. Biochem J. 1985 Sep 15;230(3):753–764. doi: 10.1042/bj2300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grdisa M., Vitale L. Types and localization of aminopeptidases in different human blood cells. Int J Biochem. 1991;23(3):339–345. doi: 10.1016/0020-711x(91)90116-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S., Murray H., Scott C. S., Turner A. J., Kenny A. J. A highly sensitive E.L.I.S.A. for endopeptidase-24.11, the common acute-lymphoblastic-leukaemia antigen (CALLA, CD-10), applicable to material of porcine and human origin. Biochem J. 1991 Sep 1;278(Pt 2):417–421. doi: 10.1042/bj2780417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Quackenbush E. J., Ronco P., Verroust P., Carrel S., Letarte M. Common acute lymphoblastic leukemia antigen expressed on leukemia and melanoma cell lines has neutral endopeptidase activity. J Clin Invest. 1989 Feb;83(2):713–717. doi: 10.1172/JCI113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H., Kanno T. Properties and activities of aminopeptidases in normal and mitogen-stimulated human lymphocytes. Biochem J. 1985 Feb 15;226(1):59–65. doi: 10.1042/bj2260059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look A. T., Ashmun R. A., Shapiro L. H., Peiper S. C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989 Apr;83(4):1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Stephenson S. L., Hryszko J., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Phase separation of synaptic membrane preparations with Triton X-114 reveals the presence of aminopeptidase N. Biochem J. 1985 Oct 15;231(2):445–449. doi: 10.1042/bj2310445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M., Ohishi N., Mutoh H., Izumi T., Bito H., Wada H., Seyama Y., Toh H., Shimizu T. Leukotriene A4 hydrolase is a zinc-containing aminopeptidase. Biochem Biophys Res Commun. 1990 Dec 14;173(2):620–626. doi: 10.1016/s0006-291x(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Orning L., Krivi G., Bild G., Gierse J., Aykent S., Fitzpatrick F. A. Inhibition of leukotriene A4 hydrolase/aminopeptidase by captopril. J Biol Chem. 1991 Sep 5;266(25):16507–16511. [PubMed] [Google Scholar]
- Orning L., Krivi G., Fitzpatrick F. A. Leukotriene A4 hydrolase. Inhibition by bestatin and intrinsic aminopeptidase activity establish its functional resemblance to metallohydrolase enzymes. J Biol Chem. 1991 Jan 25;266(3):1375–1378. [PubMed] [Google Scholar]
- Rautenberg W., Tschesche H. Aminopeptidases from human leucocytes. Hoppe Seylers Z Physiol Chem. 1984 Jan;365(1):49–58. doi: 10.1515/bchm2.1984.365.1.49. [DOI] [PubMed] [Google Scholar]
- Riemann D., Schwachula A., Hentschel M., Langner J. Demonstration of CD13/aminopeptidase N on synovial fluid T cells from patients with different forms of joint effusions. Immunobiology. 1993 Jan;187(1-2):24–35. doi: 10.1016/S0171-2985(11)80243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Vijayaraghavan J., Schmidt E. V., Masteller E. L., D'Adamio L., Hersh L. B., Reinherz E. L. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 ("enkephalinase"): direct evidence by cDNA transfection analysis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):297–301. doi: 10.1073/pnas.86.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987 Jan 1;241(1):237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderling E. Effect of Cl- on the function of the Cl- -activated arginine aminopeptidase purified from human erythrocytes. Arch Biochem Biophys. 1982 Jun;216(1):105–115. doi: 10.1016/0003-9861(82)90194-1. [DOI] [PubMed] [Google Scholar]
- Söderling E. Substrate specificities of Cl- -activated arginine aminopeptidases from human and rat origin. Arch Biochem Biophys. 1983 Jan;220(1):1–10. doi: 10.1016/0003-9861(83)90380-6. [DOI] [PubMed] [Google Scholar]
- Ulmer A. J., Mattern T., Feller A. C., Heymann E., Flad H. D. CD26 antigen is a surface dipeptidyl peptidase IV (DPPIV) as characterized by monoclonal antibodies clone TII-19-4-7 and 4EL1C7. Scand J Immunol. 1990 Apr;31(4):429–435. doi: 10.1111/j.1365-3083.1990.tb02789.x. [DOI] [PubMed] [Google Scholar]
- Vitale L., Zubanović M., Abramić M. Properties and distribution of aminopeptidase and dipeptidyl aminopeptidase III of human erythrocytes. Acta Biol Med Ger. 1981;40(10-11):1489–1495. [PubMed] [Google Scholar]
- Wetterholm A., Haeggström J. Z. Leukotriene A4 hydrolase: an anion activated peptidase. Biochim Biophys Acta. 1992 Feb 12;1123(3):275–281. doi: 10.1016/0005-2760(92)90007-i. [DOI] [PubMed] [Google Scholar]
- Wu Q., Lahti J. M., Air G. M., Burrows P. D., Cooper M. D. Molecular cloning of the murine BP-1/6C3 antigen: a member of the zinc-dependent metallopeptidase family. Proc Natl Acad Sci U S A. 1990 Feb;87(3):993–997. doi: 10.1073/pnas.87.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Li L., Cooper M. D., Pierres M., Gorvel J. P. Aminopeptidase A activity of the murine B-lymphocyte differentiation antigen BP-1/6C3. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):676–680. doi: 10.1073/pnas.88.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


