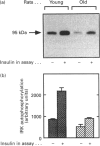
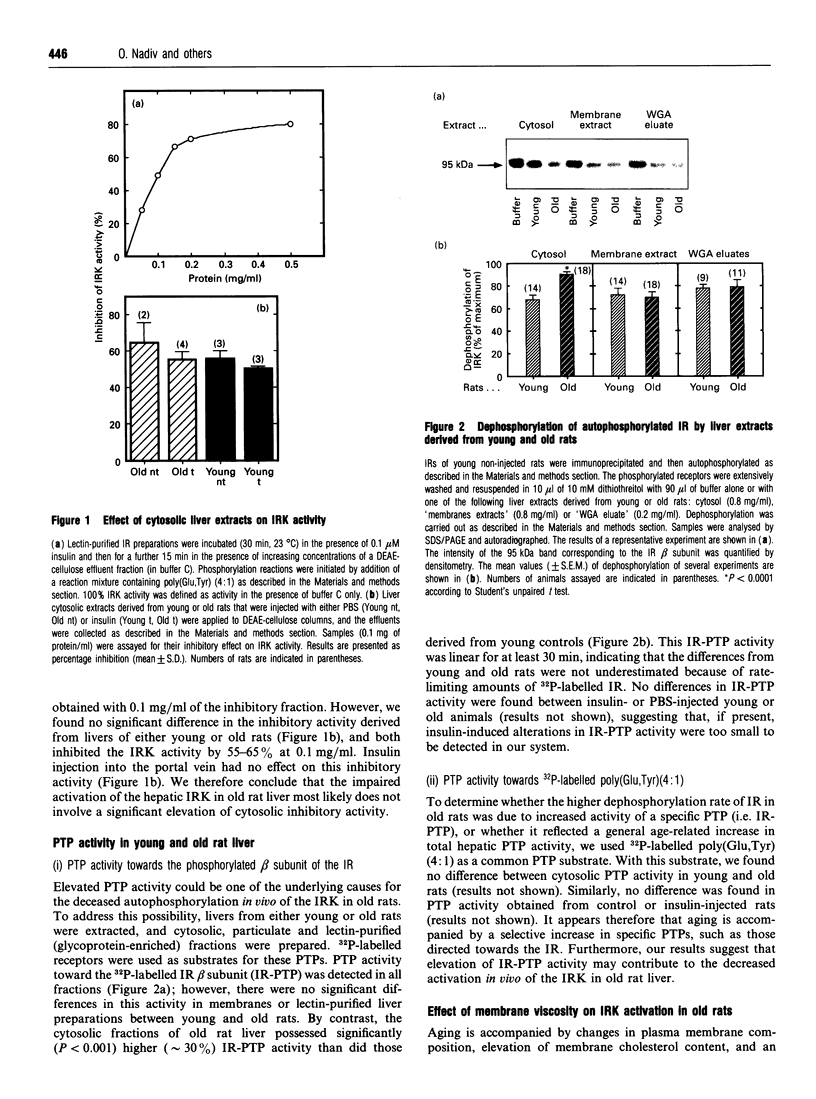
Abstract
Insulin resistance is very common in the elderly, and may be associated with glucose intolerance or frank diabetes. In previous studies we demonstrated that insulin resistance in old Wistar rats is associated with decreased autophosphorylation and activation of the hepatic insulin receptor kinase (IRK) in vivo. We now show that this defect can be reproduced in vitro, where the extent of insulin-induced activation of IRK in liver membranes of old rats was decreased by approximately 50% compared with young controls. The defect could be largely abolished after solubilization of the membranes with Triton X-100. We also show that: (a) the viscosity of membranes from the old rats was significantly (P < 0.001, n = 4) higher (by 15%) compared with young controls; (b) incubation of plasma membranes from old animals with lecithin liposomes, which lowered their cholesterol levels, partially abolished the defect in IRK activation; and (c) Triton extracts of liver membranes prepared from old rats did not interfere with the activation of IRK derived from young controls. Additionally, non-membrane components did contribute to the development of this defect. We observed a significant (approximately 30%) (P < 0.001, n = 18) elevation of cytosolic protein tyrosine phosphatase (PTP) activity directed against the beta subunit of the insulin receptor in livers of old rats. No such elevation of PTP activity could be demonstrated with synthetic substrates. Our findings are consistent with a model in which increased membrane viscosity as well as enhancement of a cytosolic PTP activity both markedly inhibit the activation in vivo of the hepatic IRK in old animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. S., Newton A. C. Inhibition of the insulin receptor tyrosine kinase by sphingosine. Biochemistry. 1991 Aug 6;30(31):7747–7754. doi: 10.1021/bi00245a011. [DOI] [PubMed] [Google Scholar]
- Auberger P., Falquerho L., Contreres J. O., Pages G., Le Cam G., Rossi B., Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989 Aug 25;58(4):631–640. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Becker A. B., Roth R. A. Insulin receptor structure and function in normal and pathological conditions. Annu Rev Med. 1990;41:99–115. doi: 10.1146/annurev.me.41.020190.000531. [DOI] [PubMed] [Google Scholar]
- Begum N., Sussman K. E., Draznin B. Differential effects of diabetes on adipocyte and liver phosphotyrosine and phosphoserine phosphatase activities. Diabetes. 1991 Dec;40(12):1620–1629. doi: 10.2337/diab.40.12.1620. [DOI] [PubMed] [Google Scholar]
- Biener Y., Zick Y. Basic polycations activate the insulin receptor kinase and a tightly associated serine kinase. Eur J Biochem. 1990 Nov 26;194(1):243–250. doi: 10.1111/j.1432-1033.1990.tb19449.x. [DOI] [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Klarlund J. K., Yagaloff K. A., Bradford A. P., Lewis R. E. Insulin receptor signaling. Activation of multiple serine kinases. J Biol Chem. 1988 Aug 15;263(23):11017–11020. [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Kolterman O. G., Griffin J., Olefsky J. M. Mechanisms of insulin resistance in aging. J Clin Invest. 1983 Jun;71(6):1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gruppuso P. A., Boylan J. M., Posner B. I., Faure R., Brautigan D. L. Hepatic protein phosphotyrosine phosphatase. Dephosphorylation of insulin and epidermal growth factor receptors in normal and alloxan diabetic rats. J Clin Invest. 1990 Jun;85(6):1754–1760. doi: 10.1172/JCI114632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S., Peraldi P., Alengrin F., Van Obberghen E. Alteration of phosphotyrosine phosphatase activity in tissues from diabetic and pregnant rats. Endocrinology. 1993 Jan;132(1):67–74. doi: 10.1210/endo.132.1.8419148. [DOI] [PubMed] [Google Scholar]
- Heffetz D., Bushkin I., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990 Feb 15;265(5):2896–2902. [PubMed] [Google Scholar]
- Heffetz D., Fridkin M., Zick Y. Antibodies directed against phosphothreonine residues as potent tools for studying protein phosphorylation. Eur J Biochem. 1989 Jun 15;182(2):343–348. doi: 10.1111/j.1432-1033.1989.tb14836.x. [DOI] [PubMed] [Google Scholar]
- Heffetz D., Zick Y. Receptor aggregation is necessary for activation of the soluble insulin receptor kinase. J Biol Chem. 1986 Jan 15;261(2):889–894. [PubMed] [Google Scholar]
- Hegner D. Age-dependence of molecular and functional changes in biological membrane properties. Mech Ageing Dev. 1980 Sep-Oct;14(1-2):101–118. doi: 10.1016/0047-6374(80)90109-8. [DOI] [PubMed] [Google Scholar]
- Hubert P., Bruneau-Wack C., Cremel G., Le Marchand-Brustel Y., Staedel C. Lipid-induced insulin resistance in cultured hepatoma cells is associated with a decreased insulin receptor tyrosine kinase activity. Cell Regul. 1991 Jan;2(1):65–72. doi: 10.1091/mbc.2.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H. U. The insulin receptor: signalling mechanism and contribution to the pathogenesis of insulin resistance. Diabetologia. 1991 Dec;34(12):848–861. doi: 10.1007/BF00400192. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Erikson R. L., Ingebritsen V. M., Ingebritsen T. S. Phosphotyrosyl-protein phosphatases. I. Separation of multiple forms from bovine brain and purification of the major form to near homogeneity. J Biol Chem. 1989 May 5;264(13):7747–7753. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi M., Jameson D. M., van der Meer B. W. Role of BBM lipid composition and fluidity in impaired renal Pi transport in aged rat. Am J Physiol. 1989 Jan;256(1 Pt 2):F85–F94. doi: 10.1152/ajprenal.1989.256.1.F85. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Czech M. P. Phospholipid environment alters hormone-sensitivity of the purified insulin receptor kinase. Biochem J. 1987 Dec 15;248(3):829–836. doi: 10.1042/bj2480829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J Clin Invest. 1989 Sep;84(3):976–983. doi: 10.1172/JCI114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerovitch J., Rothenberg P., Shechter Y., Bonner-Weir S., Kahn C. R. Vanadate normalizes hyperglycemia in two mouse models of non-insulin-dependent diabetes mellitus. J Clin Invest. 1991 Apr;87(4):1286–1294. doi: 10.1172/JCI115131. [DOI] [PMC free article] [PubMed] [Google Scholar]
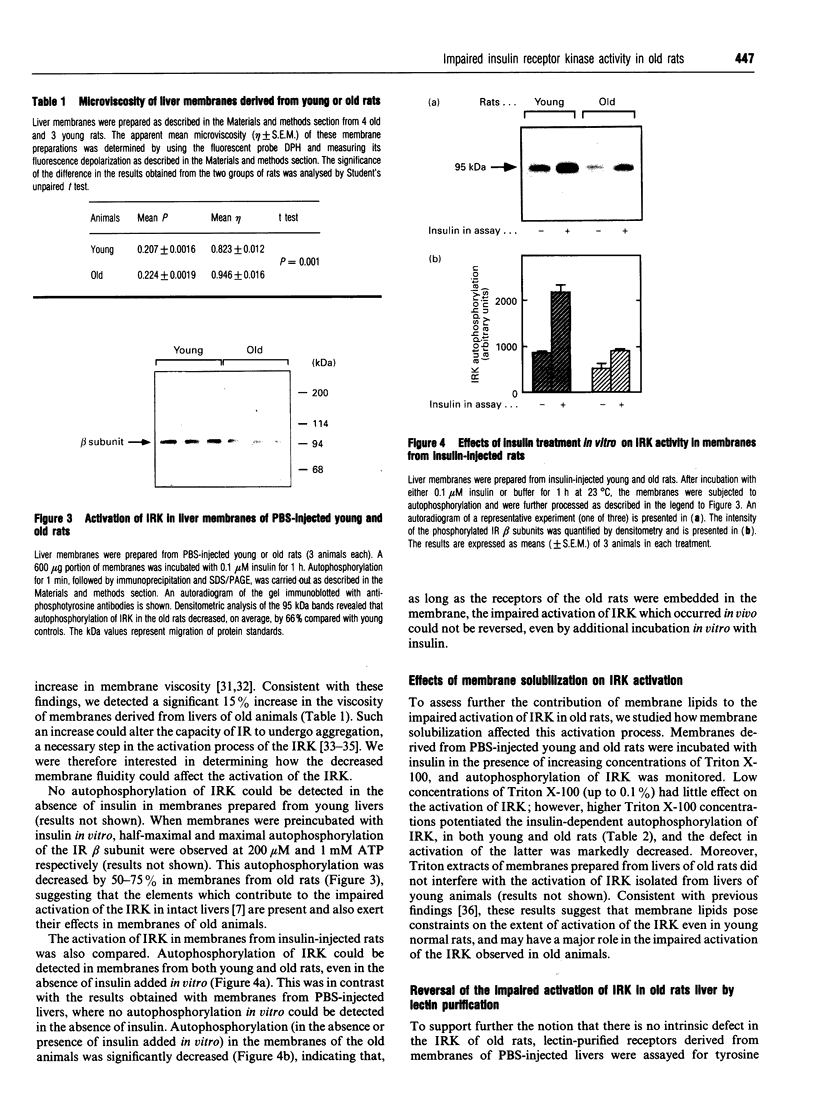
- Nadiv O., Cohen O., Zick Y. Defects of insulin's signal transduction in old rat livers. Endocrinology. 1992 Mar;130(3):1515–1524. doi: 10.1210/endo.130.3.1311243. [DOI] [PubMed] [Google Scholar]
- Nokubo M. Physical-chemical and biochemical differences in liver plasma membranes in aging F-344 rats. J Gerontol. 1985 Jul;40(4):409–414. doi: 10.1093/geronj/40.4.409. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Soos M. A., Siddle K. Monoclonal antibodies to the insulin receptor stimulate the intrinsic tyrosine kinase activity by cross-linking receptor molecules. EMBO J. 1987 Dec 20;6(13):4003–4010. doi: 10.1002/j.1460-2075.1987.tb02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
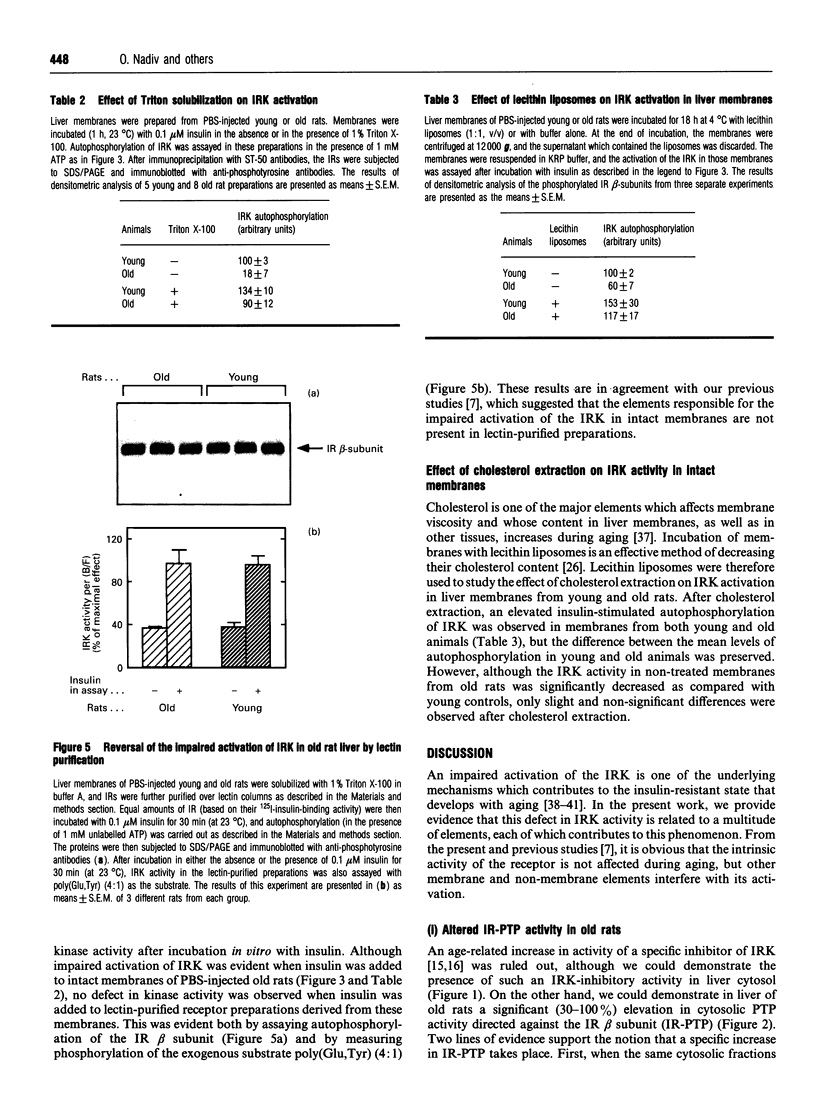
- O'Rahilly S., Moller D. E. Mutant insulin receptors in syndromes of insulin resistance. Clin Endocrinol (Oxf) 1992 Feb;36(2):121–132. doi: 10.1111/j.1365-2265.1992.tb00945.x. [DOI] [PubMed] [Google Scholar]
- Petkova D. H., Momchilova-Pankova A. B., Markovska T. T., Koumanov K. S. Age-related changes in rat liver plasma membrane sphingomyelinase activity. Exp Gerontol. 1988;23(1):19–24. doi: 10.1016/0531-5565(88)90016-2. [DOI] [PubMed] [Google Scholar]
- Petkova D. H., Momchilova A. B., Koumanov K. S. Age-related changes in rat liver plasma membrane phospholipase A2 activity. Exp Gerontol. 1986;21(3):187–193. doi: 10.1016/0531-5565(86)90072-0. [DOI] [PubMed] [Google Scholar]
- Richardson D. K., Czech M. P. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. Am J Physiol. 1978 Feb;234(2):E182–E189. doi: 10.1152/ajpendo.1978.234.2.E182. [DOI] [PubMed] [Google Scholar]
- Roome J., O'Hare T., Pilch P. F., Brautigan D. L. Protein phosphotyrosine phosphatase purified from the particulate fraction of human placenta dephosphorylates insulin and growth-factor receptors. Biochem J. 1988 Dec 1;256(2):493–500. doi: 10.1042/bj2560493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Beaudoin J. Phosphorylation of purified insulin receptor by cAMP kinase. Diabetes. 1987 Jan;36(1):123–126. doi: 10.2337/diab.36.1.123. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Minaker K. L., Pallotta J. A., Flier J. S. Characterization of the insulin resistance of aging. J Clin Invest. 1983 Jun;71(6):1581–1587. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbraccia P., Goodman P. A., Maddux B. A., Wong K. Y., Chen Y. D., Reaven G. M., Goldfine I. D. Production of inhibitor of insulin-receptor tyrosine kinase in fibroblasts from patient with insulin resistance and NIDDM. Diabetes. 1991 Feb;40(2):295–299. doi: 10.2337/diab.40.2.295. [DOI] [PubMed] [Google Scholar]
- Shiba T., Tobe K., Koshio O., Yamamoto R., Shibasaki Y., Matsumoto N., Toyoshima S., Osawa T., Akanuma Y., Takaku F. Concanavalin A-induced receptor aggregation stimulates the tyrosine kinase activity of the insulin receptor in intact cells. Biochem J. 1990 May 1;267(3):787–794. doi: 10.1042/bj2670787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Spinedi A., Rufini S., Luly P. Age-dependent changes of rat liver plasma membrane composition. Experientia. 1985 Sep 15;41(9):1141–1143. doi: 10.1007/BF01951698. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Dudley D. T., Pessin J. E., Spector A. A. Phospholipid activation of the insulin receptor kinase: regulation by phosphatidylinositol. FASEB J. 1987 Jul;1(1):55–59. doi: 10.1096/fasebj.1.1.3038645. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Taylor S. I., Cama A., Accili D., Barbetti F., Quon M. J., de la Luz Sierra M., Suzuki Y., Koller E., Levy-Toledano R., Wertheimer E. Mutations in the insulin receptor gene. Endocr Rev. 1992 Aug;13(3):566–595. doi: 10.1210/edrv-13-3-566. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Shinitzky M. Fluorescence polarization studies of lysozyme and lysozyme-saccharide complexes. J Mol Biol. 1973 Mar 15;74(4):519–531. doi: 10.1016/0022-2836(73)90044-2. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Wahnon R., Mokady S., Cogan U. Age and membrane fluidity. Mech Ageing Dev. 1989 Dec;50(3):249–255. doi: 10.1016/0047-6374(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Lipid regulation of cell membrane structure and function. FASEB J. 1989 May;3(7):1833–1842. [PubMed] [Google Scholar]
- Yegutkin G. G., Sambursky S. S., Zhitkovitch A. V., Gatsko G. G. Evaluation of age-related changes of physicochemical properties and functional activity of rat adipose plasma membranes and their possible relationship. Mech Ageing Dev. 1991 Jun 14;59(1-2):1–16. doi: 10.1016/0047-6374(91)90069-c. [DOI] [PubMed] [Google Scholar]
- Zick Y. The insulin receptor: structure and function. Crit Rev Biochem Mol Biol. 1989;24(3):217–269. doi: 10.3109/10409238909082554. [DOI] [PubMed] [Google Scholar]
- Zick Y., Whittaker J., Roth J. Insulin stimulated phosphorylation of its own receptor. Activation of a tyrosine-specific protein kinase that is tightly associated with the receptor. J Biol Chem. 1983 Mar 25;258(6):3431–3434. [PubMed] [Google Scholar]