Abstract
There is a compelling demand for approved plague vaccines due to the endemicity of Yersinia pestis and its potential for pandemic spread. Whilst substantial progress has been made, we recommend that the global funding and health security systems should work urgently to translate some of the efficacious vaccines reviewed herein to expedite clinical development and to prevent future disastrous plague outbreaks, particularly caused by antimicrobial resistant Y. pestis strains.
Content includes material subject to Crown Copyright © 2024.This is an open access article under the Open Government License (http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/).
Subject terms: Bacterial infection, Bacterial infection
Main text
Epidemiology of plague
Plague, caused by the gram-negative bacterium Yersinia pestis, is notorious for its involvement in three of the seven deadliest pandemics recorded in global history, including the recent COVID-19 pandemic. The three historic plague pandemics, the most infamous of which was the Black Death of the Middle Ages, collectively caused an estimated 200 million deaths1,2. Unfortunately, plague is still an endemic disease in parts of the world, with outbreaks being reported to the WHO from over 33 countries including Madagascar, Democratic Republic of the Congo (DRC), India, China, Peru, and occasionally, the south-western USA3. In these regions, disease is maintained by the existence of infected animal (mostly rodent) reservoirs of Y. pestis4,5.
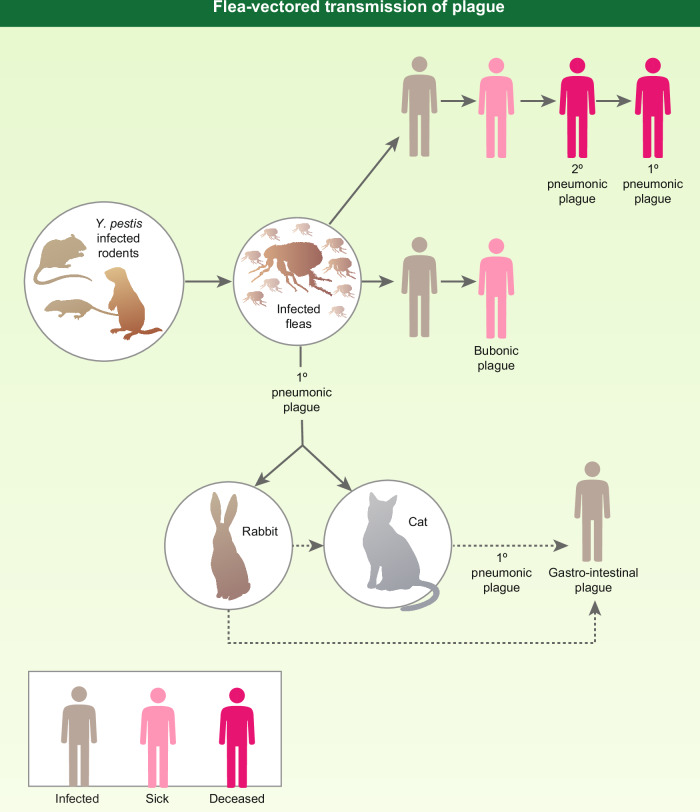
Transmission to humans is predominantly promoted by flea bite; those fleas having fed on infected rodents (Fig. 1). However, Y. pestis is an obligate parasite and even if the rat population is reduced, the organism can infect mice, prairie dogs, rabbits, and members of the cat family, including the domestic cat4–6.
Fig. 1. Flea-vectored transmission of plague.
The figure depicts various routes for the flea-vectored transmission of plague to man. The figure is reproduced from Williamson and Westlake (2019)8 with permission (License 5753521304382, Oxford University Press).
Infection through flea bite causes bubonic plague, which if undiagnosed, can develop into a septicemic infection or secondary pneumonic plague. Pneumonic plague is highly contagious requiring prompt antibiotic therapy for survival, as the mortality rate approaches 100% if untreated6–8. In pneumonic plague, Y. pestis can be transmitted to healthy individuals who are in close contact by respiratory droplets, establishing further cases of primary pneumonic plague, leading to disease outbreaks which may transform into epidemics and pandemics6.
With this epidemiology, poor living conditions augment the endemicity of plague, which requires close contact with a rodent population. However, in endemic regions such as Madagascar, the lack of an approved vaccine means that outbreaks have to be controlled by antibiotic therapy, administered to the patients and those in immediate contact with the infected individuals. Whilst timely antibiotic therapy is effective in treating the infection, case fatality rates still reached up to 8.6% during the 2017 Madagascar outbreak despite aggressive antibiotic therapy9. Additionally, there is a demonstrable risk of the development of antibiotic resistance. Indeed, antimicrobial resistant (AMR) Y. pestis strains have been identified in Peru and Madagascar10,11. Therefore, there is a clear need for a safe, effective, and licensed vaccine for use in endemic regions to control or prevent infection, as well as to protect military and civilians at large from potential biothreat attacks.
Emergence of Yersinia pestis as a dangerous pathogen
Y. pestis has evolved from the relatively mild gastrointestinal Y. pseudotuberculosis (notably serotype 1b) between 1500 and 20,000 years ago12, although archaeological evidence has suggested that the plague-causing bacterium existed long before previous estimates13.
The evolution of Y. pestis has resulted in the inactivation of genes required for an enteric lifestyle and by the acquisition of plasmids encoding new virulence factor-encoding genes14. In common with other pathogenic yersiniae (e.g., Y. pseudotuberculosis and Y. enterocolitica), Y. pestis possesses a 70-kilobase (kb) virulence plasmid designated as pYV/pCD1 that carries a Type III secretion system (TTSS) operon15,16. However, Y. pestis has acquired two additional unique plasmids, including a 9.5-kb pPCP1/pPla/pPst encoding a bacterial surface-bound protease (plasminogen activator, Pla), which has potent fibrinolytic activity1. In addition, this plasmid possesses pesticin and coagulase encoding genes which enable bacterial transmission from the flea17. The other 100–110 kb pFra/pMT1 plasmid18 codes for two important proteins, Fraction 1 (F1) antigen and a phospholipase D known as murine toxin. The F1 antigen forms a polymeric anti-phagocytic capsule around the bacteria18 whilst murine toxin has a role in preserving Y. pestis in the flea gut19. During its evolution from enteric to flea-vectored pathogen, Y. pestis has lost intestinal adhesin and invasin genes, but has retained the heme locus and possesses a number of chromosomal-encoded genes such as the ph6/psa fimbrial and attachment-invasion locus (ail) which promote colonization to the host cells19–21.
Evasion of host responses
In the process of acquiring a new mechanism of infection, Y. pestis has also activated genes which enable the pathogen to evade the defenses of its successive hosts. In purified or recombinant forms, some of these encoded gene products have provided vaccine targets and are therefore summarized here.
Y. pestis can survive and grow in the flea’s (most notably the rat flea Xenopsylla cheopis) foregut, leading to ‘blockage’ of the flea. The proper functioning of the bacterial hemin storage system is thought to play an important role in the formation of this blockage19, which during the flea bite, results in the regurgitation of a dense bolus of bacteria5 into a new host. Y. pestis expresses other genes in the flea gut such as a ‘murine’ toxin with phospholipase D activity20 and a lipopolysaccharide (LPS) core modification locus, which together are required for biofilm formation and blockage of the flea20–22. However, transcriptional analysis of Y. pestis in the flea gut has identified a wide range of additional genes, such as insecticidal-like toxin genes, which are differentially regulated such that bacteria regurgitated into a new host have increased resistance to innate immune effectors23.
Upon infection of a new mammalian host, the plague bacilli are vulnerable to phagocytosis by polymorphonuclear leukocytes (PMNs or neutrophils) and/or monocytes. The bacteria may be killed within PMNs, but can persist within monocytes and express various virulence determinants, allowing Y. pestis growth and eventual release from the monocytes24. The fibrillar adhesin pH6 antigen is induced by low phagosomal pH (4.5)25 and promotes bacterial adhesion to host cells, thereby enhancing resistance to phagocytosis26. Secretion of the F1 antigen with capsule formation is triggered by a temperature shift from 28 °C in the flea to 37 °C in humans or other mammals. The F1 capsule also plays a key role in avoiding phagocytosis27. However, non-capsulated Y. pestis retains its full capability to cause pneumonic infection in animals, while having reduced virulence during bubonic infection28.
The dominant anti-host effects are due to a temperature shift induction of the TTSS carried on the virulence plasmid pYV/pCD1. TTSS effectors, historically called Yersinia outer membrane proteins (Yops), have cytotoxic and phagocyte regulatory effects, are secreted through an injectosome after Y. pestis makes contact with the host cell, and are delivered into target cells15. The function of many of the Yops has been delineated for this well-characterized secretion system, and serves as a paradigm for other bacterial TTSS’s15. For example, the YopE protein is a cytotoxin and the YopH protein is a tyrosine phosphatase with anti-phagocytic activity29. The V (or Low calcium response V, LcrV) antigen plays a pivotal role by orchestrating intracellular Yop low calcium response protein G (LcrG) elaboration of the injectosome and then itself being delivered through this needle-like structure to be assembled as a pentamer at the tip30. Additionally, V antigen secreted from Y. pestis exerts a local immunomodulatory effect in the host by down-regulating the production of interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα)31,32.
Plasminogen activator (Pla) is another major virulence factor in Y. pestis. Pla is an outer membrane-located protease, which breaks down the physical barriers of connective tissue in the host, thus promoting the systemic dissemination of the bolus of Y. pestis injected by the flea. The requirement for Pla has driven the selection in Y. pestis of the “rough” phenotype of LPS, which lacks an O antigen33,34, a rare phenomenon amongst gram-negative bacteria which has possibly resulted from the bacterium’s transmission through the flea, but which is necessary for Pla to be functional35,36. Inactivation of the O-antigens on Y. pestis LPS exposes the LPS core, so that Y. pestis can interact with C-type lectin receptors on host macrophages, promoting its uptake, and thus accelerating bacterial dissemination in the host37. Our study has also shown that the Δpla mutant is unable to survive efficiently in murine and human macrophages, unlike the wild-type Y. pestis38.
The bacteria disseminate from the site of primary infection into draining regional lymph nodes. Within the lymph node, further growth of the bacteria accompanied by a massive inflammatory reaction leads to lymphadenopathy and the formation of buboes, typically in the groin or axillae. In the bubo, bacteria are predominantly extracellular, mainly due to the TTSS which is highly expressed in the lymph node39. An ability to proliferate in the bubo40 is enabled by the efficient and abundant iron acquisition systems possessed by Y. pestis41.
Eventually, the bacteria are disseminated by the lymphatic system, gain access to the blood stream, and colonize pulmonary tissues, which may lead to development of the pneumonic form of the disease. When left untreated, pneumonic plague induces an overwhelming septicemia which triggers septic shock in the host. However, the precise mechanisms that lead to the death of the host have not been identified but involve multi-organ failure, during which the systemic induction of nitric oxide synthase may contribute, as seen with other gram-negative septicemias42.
Whilst pigmentation (pgm)-negative strains of Y. pestis are usually avirulent and attenuated, the risk of reversion to virulence was highlighted by the fatal case of a laboratory worker who was unknowingly suffering from hemochromatosis and was exposed to the attenuated pgm- Y. pestis laboratory strain KIM. This individual developed plague and died, presumably due to his hemochromatosis-induced iron overload condition providing the infecting KIM strain, attenuated through defects in its iron acquisition ability, with sufficient iron to render it virulent43.
Early vaccines
The early use of an inactivated whole cell vaccine for plague by Haffkine between 1897 and 1935 successfully curtailed plague outbreaks in India. This was the first demonstration that components of Y. pestis, even when inactivated, could be immunogenic. Haffkine’s heat-killed whole cell (KWC) vaccine was administered to the human population in an estimated 24 million doses44 (Table 1).
Table 1.
Early generation plague vaccines
| Vaccine | Type | Doses | Route | Species tested | Protection | Type of Immune Response | Shortcomings | Years studied (Ref) |
|---|---|---|---|---|---|---|---|---|
| Haffkine vaccine | Heat-killed | 1 | s.c. | rabbits | Bubonic only | Likely Ab only | Severely reactogenic | 1897–193544 |
| Plague vaccine (USP) Or CSL vaccine |
Formalin-inactivated Heat-inactivated |
3+ 3 |
i.m. i.m. |
Mice mice |
Bubonic only | Ab | Frequent boosters, reactogenic | 1939–199945–53 |
| Live plague vaccine (EV76, EVNIIEG) | Live-attenuated | 1+ | Various $ | Mice, Rats, Guinea Pigs, NHP’s* | Both bubonic and pneumonic | Ab and CMI | Frequent boosters, Reactogenic, Virulent during iron overload | 1936–present54–57 |
Dollar sign indicates various routes include skin scarification, intradermal, sub-cutaneous (s.c.), oral (p.o) and inhalational. Asterisk indicates live plague vaccine can cause disease in African Green monkeys (AGMs). In humans, EV76 is recommended to be administered once a year. It is used in Former States of Soviet Union and regions where plague is endemic but is not approved in USA/Europe; antibodies to F1, LcrV and YscF have been detected in vaccinated humans. Commonwealth Serum Laboratories (CSL) in Australia produced heat-killed vaccine, administered in 3 doses in humans. Ab Antibody, CMI Cell-mediated immunity, GP guinea pig, NHP non-human primate, s.c. sub-cutaneous, i.m. intra-muscular.
During the 1990s, there were several commercial suppliers of the KWC vaccine against plague. Subsequently, plague vaccine USP (United States Pharmacopeia; 1939–1999), containing formaldehyde-killed bacteria, was manufactured by Cutter Laboratories, USA. In 1994, the manufacturing was transferred to Greer Laboratories Inc., USA. In 1999, the production of this vaccine was discontinued largely because of severe side effects and its protection against bubonic but limited efficacy against pneumonic plague45–52. An alternative heat-killed (KWC) vaccine was also manufactured by the Commonwealth Serum Laboratories (CSL, Australia)53 and until November 2005, was licensed for clinical use in Australia. Additionally, a Y. pestis isolate (EV76-NIIEG Y. pestis)54, which is attenuated due to deletion of the pigmentation locus (pgm), has been used as a vaccine for many years and is licensed for use in China and Russia specifically55 where plague is endemic. The vaccine can be administered by various routes; however, the vaccine is fully virulent under iron-overload conditions, i.e., in individuals with hemochromatosis56,57 (Table 1).
Virulence factors as vaccine antigens
The seminal observation in 1956 by Bacon and Burrows that Pasteurella pestis (now Y. pestis) could be anti-phagocytic in the absence of capsule, led to the identification of a new virulence antigen, which they named the V antigen58. This paved the way for subsequent research to the present day on the immunogenic and protective potential of this and other virulence factors of Y. pestis59,60. Building on the observation that the F1 antigen-containing Cutter KWC vaccine needed the addition of a recombinant V (rV) antigen to fully protect mice against pneumonic plague61, Williamson, et al. demonstrated the synergistic effect of F1 and V in combination. Whilst vaccines lacking the V antigen may protect against bubonic plague, several groups showed that the inclusion of the V antigen was an essential requirement for protection against pneumonic plague61,62.
Much work has been carried out to determine the protective potential of other antigens derived from Y. pestis in native or recombinant form in addition to F1 and V, such as Pla, a protein constituent of the injectisome known as Yersinia secretory factor F (YscF), and a range of other Yops, and their various combinations63–67. Whilst some of these imparted partial protective efficacy and are useful adjuncts in some vaccine formulations (see below), to date F1 and V remain the key proteins which individually have protective efficacy, but which in combination, are consistently synergistic and, therefore, form the core building blocks of most vaccine approaches.
Vaccines for plague
Currently, there are more than 21 candidate vaccines in the preclinical phase3. Below, we have reviewed the pre-clinical candidates (Tables 1–5) and subsequently those that are in early clinical development with a timeline (Fig. 2). The pre-clinical candidates can be broadly categorised as subunit, live attenuated, vectored (bacterial or viral), DNA, or messenger RNA (mRNA).
Table 5.
Plague vaccines tested in NHP’s or heterologous vaccination strategy
| Vaccine | Type | Adjuvant | Doses | Route | Cyno macaque efficacy | African Green monkey efficacy | Type of immune response | Years studied (Ref) |
|---|---|---|---|---|---|---|---|---|
| rF1-V | Subunit | alum | 3 | s.c. | 80% | 20% | Ab | 2007–201851 |
| LicKM-LcrV-F1 | Subunit | LickM + alum | 3 | s.c. | 100% | Not tested | Ab | 2007–2009130 |
| rF1 + rV | Subunit | alum | 2 | i.m. | 100% | Not tested | Ab | 201168 |
| rV10 | Subunit | alum | 3 | i.m. | 100%* | 33% | Ab | 201171 |
|
rAd5-YFV + rY FV $ |
Viral vector with protein boost | self | 1 + 1 | i.n.-i.m. | 100% | Not tested | Ab | 2016114 |
|
Microvesicle Bacteroides spp.) F1-V |
OMV | self | 2 | p.o./i.n. | Not tested | Not tested | Robust IgA and IgG in blood and airways | 201980 |
| Heterologous prime-boost | ||||||||
| Vaccine | Type | Adjuvant | Doses | Route | Efficacy in mice | Type of immune response | Years Studied (Ref) | |
| Ad5-YFV/LMA** | Hetero-logous | self | 1 + 1 | Both i.n. | Pneumonic & bubonic | Ab and CMI | 2021–202356 | |
Single asterisk indicates that only 50% of controls died. Double asterisk indicates that no clinical signs were observed in cynomolgus macaques or in African green monkeys; dollar sign indicates that Ad5 pre-existing immunity was induced prior to immunisation. OMV outer membrane vesicles, Ad5-YFV/LMA Ad-vectored and live attenuated, Ab Antibody, CMI cell-mediated immunity, s.c. sub-cutaneous, i.m. intra-muscular, i.n. intra-nasal, p.o. oral.
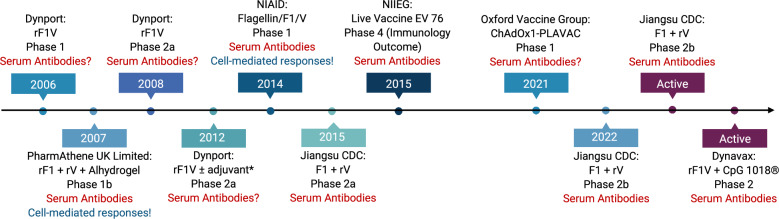
Fig. 2. Plague vaccines in clinical trials.
*Adjuvant not specified. Ages of study participants ranges from 18 to 55 years. All vaccines were given in 2–3 doses intramuscularly over a range of 6 months. The EV 76 NIIEG vaccine was given 1–4 times at intervals of 1–3 months. ?data not published; !data not conclusive.
Subunit
Many groups have now shown that immunization with the F1 and V subunit antigens provides a high degree of protection against infection caused by Y. pestis in a range of animal models68–78. The use of F1 and V in combination (F1 + V) or as a genetic fusion (F1-V) has the advantage that protection can be maintained against acapsular (F1-negative) Y. pestis strains which still retain virulence79. Many different formulations have been researched with a view to finding one that provides comprehensive protection with the least number of doses, is stable, and maintains immunogenicity when escalated up the species from mice to humans. These candidate vaccines which have been studied in much more detail are summarized in Table 2.
Table 2.
New subunit plague vaccines and adjuvants
| Vaccine | Doses | Adjuvant | Route | Species tested | Efficacy* | Immune response type | Years studied (Ref) |
|---|---|---|---|---|---|---|---|
| rF1-V | 2 | Alum | s.c. | Mice. NHP | Pneumonic | Ab | 1998–present60,69,70,85 |
| rF1 + rV | 2 | Alum | i.m. | Mice, GP, NHP | Pneumonic | Ab | 1995–201159,61,68,71 |
|
Calcium phosphate Protein-coated microcrystals (PCMC) F1V |
2 | Alum | s.c. | Mice | Pneumonic | Ab | 2018–202282 |
| Flagellin F1-V | 2 | Flagellin | i.m. |
Mice NHP $ |
Pneumonic | Ab | 2006–202074,75,124 |
| Protollin F1-V** | 2 | Protollin | i.n. | Mice | Pneumonic | Ab | 200673 |
|
Single dose F1-V Polyanhydride nanoparticles coupled with cyclic dinucleotides |
1 | STING (stimulator of interferon genes) agonist | i.n. | Mice | Pneumonic | Ab and cell-mediated (CMI) | 201976 |
| rV10 | 2 | alum | i.m. | Mice, GP, NHP | Pneumonic | Ab | 2005–201171 |
| Peptidoglycan-free OMV (Bacterial ghosts)-phage lytic system | 2 | self | s.c. |
Mice GP |
Bubonic | Ab and CMI | 202178 |
| Manganese silicate nanoparticle rF1-V10 | 2 | self | s.c. | Mice | Pneumonic | Ab and CMI | 202377 |
| Polymeric F1 + LcrV (ILB1)-R | 1 | alum | s.c. | Mice | Pneumonic | Ab | 202372 |
| Y. pseudotuberculosis-based LcrV MPLA OMV | 2 | MPLA (monophosphoryl lipid A) | i.m. | Mice | Pneumonic | Ab and CMI | 2020–202381,105 |
| Plague microencapsulated vaccine (licensed in Russia) | 2 | Alum + self | s.c. |
Mice GP NHP Humans |
Bubonic | Ab and CMI | 1983–201886 |
Asterisk indicates pneumonic infection can be via aerosol or intra-nasal. Double asterisk indicates proteosomes are non-covalently coupled to LPS. Dollar sign indicates no challenge data shown. GP Guinea pig, NHP Non-human primate, OMV outer membrane vesicles, Ab Antibody, CMI Cell-mediated immunity, s.c. sub-cutaneous, i.m. intra-muscular, i.n. intra-nasal.
Adjuvants used in preclinical rF1V vaccine development studies include the toll-like receptor 5 (TLR5) ligand flagellin and protollin73–75. Packaged in a polyanhydride nanoparticle with cyclic dinucleotide and delivered as a single dose intranasally, rF1V protected mice against pneumonic plague76. A truncated form of rV (rV10) has also been shown to be protective, as has rV10 in manganese silicate nanoparticles71,77. Similarly, the peptidoglycan-free outer membrane vesicles (OMV) with a phage lytic system has been demonstrated to be efficacious78, as have microvesicles derived from human commensal gut bacteria for immunization with V antigen80 or OMV’s from Y. pseudotuberculosis81. A dry formulation of rF1+rV delivered on calcium phosphate-decorated microparticles demonstrated enhanced immunogenicity and efficacy82.
A study has shown that the polymeric form of F1 led to rapid protective humoral immune response by activating innate-like B1b cells83 (Table 2), and further observations suggested that this activation was unaffected by the presence of the V antigen in an admixture of F1 and V83. Recent research has evaluated the impact of the administration of synthetic immunomodulating peptides on the survival of mice and guinea pigs subsequently exposed to virulent Y. pestis84. Administered in three doses prior to animal challenge, two immunomodulators were found to have a positive impact on survival; these were an azoximer bromide (polyoxidonium) and rIFNγ84. Another study has shown that co-formulation of the rF1-V vaccine with recombinant human (rhIL2) and/or recombinant murine GM-CSF in alhydrogel enhanced immunogenicity and efficacy against a lethal aerosol challenge in mice85.
In April 2024, the Russian state regulator granted a marketing authorization86 for a single sub-cutaneous dose microencapsulated molecular plague vaccine (PMMM) comprising 25–30 µg each of rF1 and rV in 4–6 mg polylactide, with the excipients polyvinyl alcohol, alhydrogel, polyvinyl pyrrolidine, polysorbate in phosphate buffered saline and containing 30–60 µg thiomersal.
Live attenuated
The live attenuated vaccine (LAV) strain EV76-NIIEG has been used for human vaccination in Russia and China for many years to prevent or curtail outbreaks of plague3. Recently, the experimental evaluation of polyoxidonium co-administered with EV76 in a murine model has been shown to improve efficacy87.
In addition to the live vaccine strain EV76, various deletion mutants of Y. pestis CO92 have been demonstrated to be efficacious in rodent models of pneumonic plague88–91 (Table 3). Among these, LMA and LMP mutants (deleted for genes encoding Braun lipoprotein [Lpp], methylacyl transferase B [MsbB], and either Attachment-invasion locus [Ail] or Plasminogen-activating protease [Pla], were of note, as they triggered robust humoral and cell-mediated immune responses in mice and were eliminated from the animals within 12–24 h88–91. Importantly, these mutants remained avirulent under iron-overload conditions56. Further, a heterologous prime-boost strategy using one dose each of LMA or LMP and replication-defective adenovius5-based three component vaccine containing genes for YscF, F1, and LcrV (Ad5-YFV) administered in any order was highly efficacious with complete protection in mice in a pneumonic plague model56 providing safety and combined benefits of subunit and live-attenuated vaccines (Table 5). Likewise, EV76 vaccine deleted for Pla92 has shown promise. Recent work has also addressed the possibility of further attenuation of Y. pestis to serve as a vaccine93. The two most protective vaccine candidates were Y. pestis CO92 mutants that were either cured for the pgm locus and the pPst plasmid or deleted for the yscN gene. These mutants completely protected BALB/c mice against subcutaneous and aerosol challenge with Y. pestis93–95 (Table 3).
Table 3.
New generation live- attenuated plague vaccines
| Vaccine | Doses | Mutation | Route | Species tested | Safety shown in immuno-compromised models | Efficacy | Type of immune response | Years studied (Ref) |
|---|---|---|---|---|---|---|---|---|
| Y. pestis CO92 ΔLMA* | 1–2 | lpp, msbB, ail | i.n. or i.m. | Mice, rats | Rag1 KOO/iron overload** | pneumonic | Ab and CMI | 201588–91 |
| Y. pestis CO92 ΔLMP | 1–2 | lpp, msbB, pla | i.m. | Mice, rats | safe | pneumonic | Ab and CMI | 201688 |
| Y. pestis EV76-B-SHU Δpla | 3 | pgm, pla | i.t. or s.c. | mice | Not tested | pneumonic | Ab and CMI | 202092 |
| Y. pestis CO92 ΔpgmΔpPst | 1–2 | pPgm, pPst (pla) | s.c. | mice | Not tested | pneumonic | Ab and CMI | 202193 |
| Y. pestis CO92 ΔyscN | 1–2 | yscN | s.c. | mice | Not tested | Bubonic and pneumonic | Ab and CMI | 202193 |
Single asterisk indicates no clinical symptoms observed in cynomolgus macaques or African Green monkeys (unpublished), double asterisk indicates avirulent under iron overload conditions. Ab Antibody, CMI Cell-mediated immunity, s.c. sub-cutaneous, i.m. intra-muscular, i.n. intra-nasal, i.t. intra-tracheal.
Vectored
Since the potential to harness rDNA technology to produce vaccines in the 1980s, many more candidate plague vaccines have been pursued45,46,52,96–116, including the use of attenuated bacterial or viral vectors to deliver antigens derived from Y. pestis. Vectors being evaluated include: Salmonella, Yersinia pseudotuberculosis, Lactobacillus, adenovirus, vesicular stomatitis virus, and vaccinia virus. Some of these well-studied vaccine candidates have been summarized in Table 4.
Table 4.
DNA and bacterial and viral-based, as well as mRNA-based plague vaccines
| Vaccine | Type | Doses | Route | Species tested | Efficacy | Immune response | Years studied (Ref) |
|---|---|---|---|---|---|---|---|
| DNA F1-V | DNA vaccine | Up to 6 | i.m. | Mice | pneumonic | Ab & CMI | 1999–201245,119 |
| Ad5-F1 + Ad5-LcrV | Adenoviral vector | 2 | i.m. | Mice | pneumonic | both | 2006–2010116,118 |
| Ad5-YFV | Adenoviral vector | 2 | i.n. | Mice, NHP | pneumonic | both | 2016–2023113,114 |
| T4-phage | Prokaryotic viral vector | 2 | i.m. | Mice, rats | pneumonic | both | 2013–2023112 |
| S. Typhimurium expressing plague antigens | Bacterial vector | 1–2 | Mostly p.o. | mice | pneumonic | both | 1996–2016107–109 |
| S. Typhi expressing plague antigens | Bacterial vector | 1–3 | i.n. | mice | Bubonic, septicaemic | both | 2004–2009106 |
| Lactiplantibacillus plantorum expressing lcrV | Bacterial vector | 3* | p.o. | mice | Not tested | both | 201145 |
| F1-mRNA-LNP | mRNA-LNP | 1 | i.m. | mice | bubonic | both | 2023120 |
| Y. pseudotuberculosis producing F1 | Bacterial vector | 1+ | s.c or p.o. | mice | Bubonic, pneumonic | both | 2008–2020101–104 |
| Self-amplifying mRNA(F1 + lcrV) | mRNA-LNP | 2 | i.m. | mice | bubonic | both | 2023121 |
| F. tularensisΔcapB + F1-LcrV/PA | Bacterial vector | 2 | i.m., i.n. | mice | Respiratory infection | both | 2018111 |
Asterisk indicates that each dose consisted of 2x daily administrations for 3–4 days. Ab Antibody, CMI Cell-mediated immunity, s.c. sub-cutaneous, i.m. intra-muscular, i.n. intra-nasal, p.o. oral.
A common advantage of these vectors is that because they are live, but replication-deficient, only 1 or 2 doses of vaccine may be required to achieve protective immunity. A second advantage is that these vectored vaccines can be multivalent, expressing antigens from different pathogens and can deliver these antigens intracellularly, mimicking infection and inducing appropriate immunity. All of these vaccine vectors require an in vivo promoter to switch on the expression of a heterogenous antigen(s) to induce an immune response. The efficiency of the promoter and the molecular size of the expressed protein-encoding genes, together with need for post-transcriptional modifications such as glycosylation, determine the level and potency of the expressed vaccine antigens. Potential disadvantages of live vaccine vectors are the necessity of stable attenuation, the risk of use in immunocompromised individuals, the possibility of inducing immunity, or pre-existing immunity to the vector itself; however, the latter can be overcome by modification of the vector or by employing a heterologous prime-boost approach to prevent reduced responses on repeated use of the same vector99,100 as we have recently shown52. Further, our study in non-human primates showed that inducing pre-existing antibodies to Ad5 did not alter protective immune responses in a pneumonic plague model114.
Bacterial
A substantial amount of research has been devoted to the development of Y. pseudotuberculosis as a vaccine for plague by deleting three essential virulence factors (High Pathogenicity Island, pH6 antigen, and YopK toxin) and by the insertion of the caf operon into the chromosome, allowing the production of an F1 pseudocapsule101–104. A Y. pseudotuberculosis construct (VTnF1) modified to maximize stability was immunogenic and efficacious against pneumonic plague in mice after a single oral dose102, and generated humoral and cell-mediated immune responses103. Subsequently, the VTnF1 vaccine has been shown to be effective in mice after subcutaneous injection and protects fully against injected (104 LD50) or 80% of animals against aerosolized (3300 LD50) Y. pestis CO92104 (Table 4).
Likewise, outer membrane vesicles (OMV) produced from a mutated version of Y. pseudotuberculosis expressing V and a modified version of LPS have been shown to be protective in mice against pneumonic plague105 (Table 4).
There has also been substantial research investment in Salmonella Typhi as a vaccine vector, particularly with its potential as an oral vaccine for plague. Early studies showed that the successful carriage by S. Typhimurium of the F1-encoding plasmid resulted in F1 protein secretion by S. Typhimurium, with visualization of the capsule surrounding the bacteria106. However, whilst immunogenicity and efficacy were achieved, sustaining the vector in vivo to retain plasmids with sufficient gene expression over time without causing salmonellosis, has been an enduring challenge107,108. More recently, combinations of F1, Psn (pesticin receptor), and V antigen delivered orally to mice using mutant strains of S. Typhimurium have provided 100% protection against subcutaneous challenge with 570 LD50 of Y. pestis CO92, but only 40-60% efficacy against 50 LD50 of aerosolized Y. pestis CO92109. Moreover, S. Typhimurium deleted for the genes lpp and msbB and used to express F1, LcrV, a combination of F1 and LcrV, and a combination of YscF and YopD, protected mice against Y. pestis CO92 infection in a pneumonic plague model45,110.
Expression of genes encoding F1 and LcrV of Y. pestis and protective antigen (PA) of Bacillus anthracis in a Francisella tularensis LVS vaccine strain provided protection to mice against all three Tier-1 select agents, raising the prospect of a polyvalent biodefense vaccine111 (Table 4).
Bacteriophage T4 serves as an excellent nanoparticle platform to deliver plague immunogens (F1 and V as well as PA antigen of B. anthracis). Both mice and rats immunized with T4 phages without any adjuvant and harboring Y. pestis and B. anthracis immunogens were protective against pneumonic plague and lethal toxin intoxication when administered sequentially or simultaneously112 (Table 4).
Viral
Recent preclinical studies have shown that vaccination of mice with Ad5-YFV provided complete protection to mice in a pneumonic plague model when challenge occurred with the F1-minus strain of Y. pestis CO92113. This is when compared to animals that were vaccinated with the monovalent, Ad5-LcrV-based vaccine, and challenged with the F1-minus strain of Y. pestis CO92 where anti-F1-antibodies were rendered ineffective113. This Ad5-YFV vaccine resulted in robust humoral and cell-mediated immune responses114. The above vaccine also provided 100% protection to Cynomolgus macaques at a very high challenge dose of Y. pestis CO92 administered by the aerosol route114 (Tables 4 and 5). An earlier version of Ad5-based vaccine harbored genes for F1 and LcrV and was shown to be protective in a murine pneumonic plague model115,116.
A chimpanzee adenovirus vector (ChAdOX1) vaccine expressing F1 and V has been developed by the Oxford Vaccine Group. The ChAdOX1 vector is a replication-deficient adenoviral vector based on the simian adenovirus type Y25, originally chosen to avoid pre-existing adenovirus immunity in the human population117. The phase 1 clinical trial started on this ChAdOX1 plague vaccine in 2021118 (Fig. 2).
DNA
DNA-based plague vaccines comprising F1 and LcrV have also been tested and found to be immunogenic and protective (Table 4). In an earlier study, we have shown that mice immunized with plasmid vectors containing genes for F1, LcrV, with a gene for LT (heat-labile enterotoxin as an adjuvant) were protective against pneumonic plague45. In this report, mice were immunized with recombinant plasmids coated with 1.6-μm gold particles and shot with the gene gun on the ears. The animals were immunized on days 0 and 3 months before intranasal challenge after 8 months following the last boost with Y. pestis CO9245.
A DNA vaccine designed to protect against both anthrax and plague was evaluated in mice119. DNA constructs comprising fusions of V with a truncated anthrax lethal factor (LF) or LF with F1 or V alone, were coated to gold nanoparticles and delivered by gene gun to A/J mice and were shown to protect fully against challenge 21days later wth aerosolized B. anthracis and to 80% against aerosolised Y. pestis119.
mRNA
More recently, mRNA technology has been extended to produce candidate plague vaccines. An mRNA vaccine expressing a circularly permutated form of F1 delivered in lipid nanoparticles (LNP) protected (100%) of mice against bubonic disease after only a single dose120, whilst a self-amplifying mRNA LNP vaccine expressing both F1 and V was immunogenic in 2 doses and also protected outbred mice against a recent clinical isolate of Y. pestis from Madagascar in a bubonic plague model121. Both of these vaccines induced humoral and cell-mediated immune responses (Table 4) and show promise for future development, allowing a very flexible platform into which additional or modified RNA could be added, if needed. Furthermore, rapid advances in large scale manufacturing and formulation achieved for mRNA vaccines for SARS-CoV-2 are now readily transferrable to the plague application122.
Translation to clinical development
Some vaccine approaches discussed above have transitioned to early clinical development (Fig. 2). Currently, a formulation of recombinant F1V (rF1V) in alum, supplemented with CpG 1018 is in Phase 2 clinical trial (Dynavax, USA)3. This form of CpG has already been incorporated in a Hepatitis B vaccine and approved by the FDA for clinical use in adults123. Also in phase 2 clinical trial is a formulation of native F1 with rV in alum (Lanzhou Institute and Jiangsu Provincial CDC, China)3. The third subunit vaccine currently in Phase 1 clinical trial comprises rF1V adjuvanted with flagellin (National Institute of Allergy and Infectious Diseases, USA)3,124.
The EV76-NIIEG vaccine is still approved only in China and Russia where it is in Phase 4 clinical trial. Since 2002, there have been vaccination campaigns with EV76 in 16-18 provinces of Mongolia by the National Centre for Zoonotic Diseases, Ulaanbaatar, Mongolia3. In a vaccination campaign report from plague-endemic foci in Mongolia, an adverse event rate of 7.3% with a 5.6% breakthrough in protection has been reported3. In October 2023, there was an ongoing campaign with EV76 in Mongolia in response to an outbreak of plague3.
Strategies to gain evidence of vaccine efficacy
As with nearly all clinical prophylaxes or therapies, the pathway to regulatory approval is time-consuming, expensive and difficult, requiring evidence (direct, indirect or deduced) of human efficacy. This is especially challenging for vaccines against Tier-1 select agents such as Y. pestis, as human challenge studies are unethical. Further, due to the endemic nature of the disease, the number of infected patients is not large enough to draw meaningful conclusions on vaccine efficacy. Here we review the strategies available to demonstrate vaccine efficacy for plague.
Animal data to support licensing
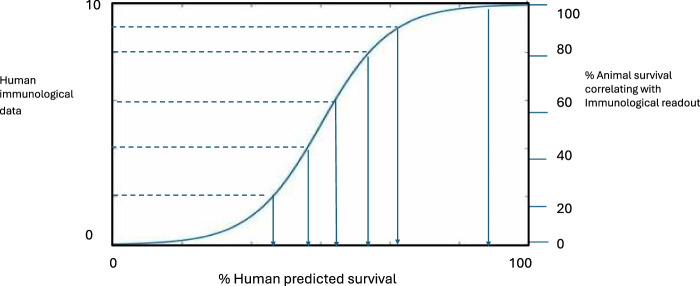
Because of the pathogenicity of Y. pestis, its potential for epidemic spread, and the unpredictable nature and size of regional plague outbreaks, the feasibility of Phase 3 trials, whether preventive or reactive in nature, is under discussion3. Even in Madagascar, where the plague season is well known, the number of cases involved varies, so a Phase 3 trial may not be sufficiently powered, unless successive seasons are used. Pathways to licensure may therefore comprise the scrutiny of immunogenicity and efficacy data generated in animal models under Good Laboratory Practice (GLP) using the FDA’s Animal rule125 with the human immunogenicity data generated in clinical trials (i.e. immunobridging)126 (Fig. 3).
Fig. 3. Immunobridging to predict vaccine efficacy in man.
The figure depicts the use of percentage survival in vaccinated animals, which correlates with an immunological readout(s), to compare with the same immunological readout determined in a clinical trial, to predict vaccine efficacy in human.
Evidence of efficacy could then be gained post-licensure. There is precedent for this with the Marketing Authorization under exceptional circumstances of an Ebola vaccine by the European Medicines Agency on the basis of human serum antibody data127. The FDA also has an accelerated approval pathway but cautions that the bar for approval of a vaccine for the pneumonic indication would be set higher than for the bubonic indication3.
Immunobridging from animal models to human
The vaccine approaches reviewed here have predominantly been screened in mice (outbred as well as inbred)128, with some in Brown Norway rats112,129 and a few in NHPs48,68,69,114,130 all of which are authentic models for plague and evaluate both antibody and cell-mediated immunity131. The most consistent NHP model is the cynomolgus macaque3,114. However, increasing diversity in response occurs with escalation up to NHPs and the human population132. Table 5 summarises the studies which have been performed in NHPs to determine the efficacy of vaccines which are in preclinical development.
Immune correlates of protection and surrogate markers of efficacy
Many researchers have now shown that antibody titers to the F1 and V proteins correlate with protection against bubonic and pneumonic plague in a range of animal models66,68,69, but the induction of cell-mediated immunity (CMI)131,132 and particularly a balanced Th1/Th2 response56,93,103,133,134 provides an optimal strategy for protection. The observation, inter alia, that mice immunized with the rF1-V vaccine and depleted of TNFα and IFNγ just prior to challenge, had poor survival compared with immunized controls which were not depleted135 indicated key roles for these Th1 cytokines in the development of protective immunity and these cumulative data have spurred the formulation of vaccine candidates which induce appropriately balanced immunity.
To enable effective immunobridging of animal data to human, it is preferable that researchers and developers use similar approaches to the measurement of antibody and cell-mediated responses3,126,132. The assay of specific antibody titers by quantitative ELISA (including the species-agnostic BRIDGE ELISA)69 is clearly important and provides a convenient surrogate marker of efficacy in the clinic.
Of equal importance is to assess cell-mediated immunity by ex vivo recall assay on animal tissue or human whole blood samples (by ELIspot or by flow cytometry)8,103,136 to determine the establishment of immune memory, and hence the need and spacing of booster doses. The ability also to assay for the induction of functional antibodies has been facilitated by the development of neutralizing monoclonal antibodies, particularly to V, enabling the development of competitive ELISAs82,137–139, which may be important aids in the down-selection of promising candidates in research. There is also ongoing effort to establish human reference serum for plague to provide an international standard as a reference point for serological surrogate markers of efficacy and thus to enable vaccine development140.
The WHO has published a draft target product profile (TPP) for a future plague vaccine, which sets out the qualifying criteria in terms of schedule, administration route, presentation, target efficacies in reactive and preventive modes, stability, and coverage, which would be applied to any plague vaccine candidate141.
Future prospects
As highlighted in this review, there are some very promising vaccine candidates in the development pipeline with the potential to prevent plague in vulnerable populations. Here, we have also highlighted the epidemic potential of this disease and of Y. pestis, which in the absence of an approved vaccine, remains a serious biothreat. Seasonal outbreaks in Madagascar and other endemic regions cause fatalities every year. The potential for climate change to enhance this human vulnerability to plague in endemic regions or beyond is also being closely monoitored142,143. Climate change has already affected other zoonoses by extending the vector species to cause outbreaks of chikungunya and zika viruses in Central and South America144,145.
Aside from the difficulties of achieving statistically valid evidence of human efficacy, candidate vaccines may also fail because their manufacturing cannot be achieved at scale due to expense or feasibility. Thus, these promising candidate vaccines for plague are vulnerable to languish in the ‘valley of death’ without sustained and sufficient funding for clinical development and manufacturing at scale. Post COVID-19, the WHO has recently commented that ‘despite some recent progress, public heath vaccines are not available in all global regions and vaccines which have been prioritized by the WHO are not being developed or fully invested in, due to limited profit potential.’146 The Immunization Agenda 2030 endorsed by the WHO has a goal to reduce the incidence of, or to prevent, epidemics caused by vector-borne diseases by 2030147. These goals seem particularly relevant to plague prevention.
To date, there has been a significant investment in time and money in the research and early development of new plague vaccines. It is to be hoped that all the R&D activity on plague vaccines, which emerged after the decline in use of KWC vaccines, will be sustained and together with the regulatory pathways currently being mapped out, will lead to the approval of some new, safe, and fully efficacious vaccines to reduce disease prevalence. We recommend that global funding and health security systems take ambitious action to realize the potential of this investment in an approved vaccine(s) for plague.
Supplementary information
Acknowledgements
E.D.W. would like to thank Prof. Tim Atkins, Prof. Riccardo d’Elia and Dr. Tom Laws for their review of the draft. Funding through the NIH (AI064389, AI153524 and AI071634) awarded to A.K.C. is gratefully acknowledged. Funding for PBK through an NIH T32 AI179595 postdoctoral fellowship in the Antimicrobial Resistance Training Program of the Texas Medical Center (AMR-TPT) is also acknowledged.
Author contributions
E.D.W. and A.K.C contributed equally to the preparation of the manuscript. All authors have read and approved the manuscript. P.B.K., E.K.H., B.H.N., and J.S. performed literature search, prepared tables and Fig. 2 and edited the review.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
E. Diane Williamson, Email: LHPPTN@dstl.gov.uk.
Ashok K. Chopra, Email: achopra@utmb.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-00958-1.
References
- 1.Brubaker, B. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol Rev.4, 309–324 (1991). 10.1128/CMR.4.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollitzer, R. Plague. World Health Organ Monogr. Ser.22, 1–698 (1954). [Google Scholar]
- 3.Global consultation on Plague Vaccines, WHO https://www.who.int/news-room/events/detail/2023/10/12/default-calendar/global-consultation-on-plague-vaccines (2023).
- 4.Doll, J. M. et al. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg.5, 109–114 (1994). 10.4269/ajtmh.1994.51.109 [DOI] [PubMed] [Google Scholar]
- 5.Perry, R. D. & Fetherston, J. D. Yersinia pestis—etiologic agent of plague. Clin. Microbiol Rev.10, 35–66 (1997). 10.1128/CMR.10.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler T. Plague and Other Yersinia Infections. (New York, Plenum Press, 1983).
- 7.Prevention of plague: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal Wkly. Rep.45, 1–15 (1996). [PubMed]
- 8.Williamson, E. D. & Westlake, G. E. Vaccines for emerging pathogens: prospects for licensure. Review series on Vaccines for emerging pathogens: from research to the clinic Part 1. Clin. Exp. Immunol.198, 170–183 (2019). 10.1111/cei.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randremanana, R. et al. Epidemiological characteristics of an urban plague epidemic in Madagascar, August-November, 2017: an outbreak report. Lancet Infect. Dis.19, 537–545 (2019). 10.1016/S1473-3099(18)30730-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guiyoule, A. et al. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis.7, 43–48 (2001). 10.3201/eid0701.010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galimand, M., Carniel, E. & Courvalin, P. Resistance of Y. pestis to antimicrobial agents. Antimicrob. Agents Chemother.50, 3233–3236 (2006). 10.1128/AAC.00306-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achtman, M. et al. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl Acad. Sci. USA96, 14043–14048 (1999). 10.1073/pnas.96.24.14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poinar G. A new genus of fleas with associated microorganisms in Dominican amber. J.Med. Entomol. 126 10.1093/jme/tjv (2015). [DOI] [PubMed]
- 14.Parkhill, J. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature413, 523–552 (2001). 10.1038/35097083 [DOI] [PubMed] [Google Scholar]
- 15.Cornelis, G. R. et al. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev.62, 1315–1352 (1998). 10.1128/MMBR.62.4.1315-1352.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, G. R. et al. The Yersinia yop regulon. Mol. Microbiol3, 1455–1459 (1989). 10.1111/j.1365-2958.1989.tb00129.x [DOI] [PubMed] [Google Scholar]
- 17.Mikula K. M., Kolodziejczyk R., Goldman A. Yersinia infection tools—characterization of structure and function of adhesins. Frontiers in Cellular and Infection Microbiology2, article 16; 10.3389/fcimb.2012.00169 (2013). [DOI] [PMC free article] [PubMed]
- 18.Du, Y., Rosqvist, R. & Forsberg, A. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun.70, 1453–146 (2002). 10.1128/IAI.70.3.1453-1460.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinnesbusch, B. J., Perry, R. D. & Schwan, T. G. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science273, 367–370 (1996). 10.1126/science.273.5273.367 [DOI] [PubMed] [Google Scholar]
- 20.Hinnebusch, B. J. et al. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science296, 733–735 (2002). 10.1126/science.1069972 [DOI] [PubMed] [Google Scholar]
- 21.Hinnebusch, B. J. Transmision factors: Yersinia pestis genes required to infect the flea vactor of plague. Adv. Exp. Med. Biol.529, 55–62 (2003). 10.1007/0-306-48416-1_11 [DOI] [PubMed] [Google Scholar]
- 22.Darby, C., Ananth, S. L., Tan, L. & Hinnebusch, B. J. Identification of gmhA, a Yersinia pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect. Immun.73, 7236–7242 (2005). 10.1128/IAI.73.11.7236-7242.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadyvaloo, V. et al. Transit through the Flea Vector Induces a pretransmission innate immunity resistance phenotype in Yersinia pestis. PLoS Pathog.6, e1000783 (2010). 10.1371/journal.ppat.1000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavanaugh, D. C. & Randall, R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol.83, 348–363 (1959). 10.4049/jimmunol.83.4.348 [DOI] [PubMed] [Google Scholar]
- 25.Lindler, L. E., Klemper, M. S. & Straley, S. C. Yersinia pestis pH 6 antigen: Genetic, biochemical and virulence characterisation of a protein involved in the pathogenesis of bubonic plague. Infect. Immun.58, 2569–2577 (1990). 10.1128/iai.58.8.2569-2577.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, X.-Z. & Lindler, L. E. The pH6 antigens is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsular antigen. Infect. Immun.72, 7212–7219 (2004). 10.1128/IAI.72.12.7212-7219.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams, R. C., Gewurz, H. & Quie, P. G. Effects of fraction 1 from Yersinia pestis on phagocytosis in vitro. J. Infect. Dis.126, 235–241 (1972). 10.1093/infdis/126.3.235 [DOI] [PubMed] [Google Scholar]
- 28.Sha, J. et al. Characterization of an F1 deletion mutant of Yersinia pestis CO92, pathogenic role of F1 antigen in bubonic and pneumonic plague, and evaluation of sensitivity and specificity of F1 antigen capture-based dipsticks. J. Clin. Microbiol.49, 1708–1715 (2011). 10.1128/JCM.00064-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straley, S. C., Skrzypek, E., Plano, G. V. & Bliska, J. B. Yops of Yersinia spp. pathogenic for humans. Infect. Immun.61, 3105–3110 (1993). 10.1128/iai.61.8.3105-3110.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller, C. A. et al. The V-antigens of Yersinia forms a distinct structure at the tip of injectisome needles. Science310, 674–676 (2005). 10.1126/science.1118476 [DOI] [PubMed] [Google Scholar]
- 31.Nakajima, R. & Brubaker, R. R. Association between virulence of Yersinia pestis and supression of gamma interferon and tumor necrosis factor alpha. Infect. Immun.61, 23–31 (1993). 10.1128/iai.61.1.23-31.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima, R., Motin, V. L. & Brubaker, R. R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunisation. Infect. Immun.6, 3021–3029 (1995). 10.1128/iai.63.8.3021-3029.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skurnik, M., Peippo, A. & Ervela, E. Characterization of the O antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol. Microbiol.37, 316–330 (2000). 10.1046/j.1365-2958.2000.01993.x [DOI] [PubMed] [Google Scholar]
- 34.Prior, J. L. et al. The failure of different strains of Yersinia pestis to produce lipopolysaccharide O antigen under different growth conditions is due to mutations in the O-antigen gene cluster. FEMS Microbiol Lett.197, 229–233 (2001). 10.1111/j.1574-6968.2001.tb10608.x [DOI] [PubMed] [Google Scholar]
- 35.Suomalainen, M. et al. Temperature-induced changes in the lipopolysaccharide of Yersinia pestis affect plasminogen activation by the pla surface protease. Infect. Immun.78, 2644–2652 (2010). 10.1128/IAI.01329-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kukkonen, M. et al. Lack of O-antigen is essential for plasminogen activation by Yersinia pestis and Salmonella enterica. Mol. Microbiol.51, 215–225 (2004). 10.1046/j.1365-2958.2003.03817.x [DOI] [PubMed] [Google Scholar]
- 37.Kun, Y. et al. Yersinia pestis Interacts with SIGNR1 (CD209b) for promoting host dissemination and infection. Front. Immunol.10, 96 (2019). 10.3389/fimmu.2019.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Lier C. J. et al. Further characterization of a highly attenuated Yersinia pestis CO92 mutant deleted for the genes encoding Braun lipoprotein and plasminogen activator protease in murine alveolar and primary human macrophages. Microb. Pathog. 80, 27–38 10.1016/j.micpath.2015.02.005. [DOI] [PMC free article] [PubMed]
- 39.Sebbane, F. et al. The Yersinia pestis caf1M1A1 fimbrial capsule operon promotes transmission by flea bite in a mouse model of bubonic plague. Infect. Immun.77, 1222–1229 (2009). 10.1128/IAI.00950-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siggins, M. K. & Sriskandan, S. Bacterial Lymphatic Metastasis in Infection and Immunity. Cells2022, 33 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaaban, T., Mohsen, Y., Ezzeddine, Z. & Ghssein, G. Overview of Yersinia pestis Metallophores: Yersiniabactin and Yersinopine. Biology12, 598 (2023). 10.3390/biology12040598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul-Clark, M. J. et al. Differential effects of Gram-positive versus Gram-negative bacteria on NOSII and TNF in macrophages: role of TLRs in synergy between the two. Brit J. Pharm.148, 1067–1075 (2006). 10.1038/sj.bjp.0706815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anon. Fatal Laboratory-Acquired Infection with an Attenuated Yersinia pestis Strain. Morbidity Mortal. Wkly. Rep. (MMWR)60, 201–205 (2011). [PubMed] [Google Scholar]
- 44.Hawgood, B. J. & Haffkine, W. M. CIE (1860–1930): Prophylactic vaccination against cholera and bubonic plague in British India. J. Med. Biogr.1, 9–19 (2007). 10.1258/j.jmb.2007.05-59 [DOI] [PubMed] [Google Scholar]
- 45.Rosenzweig, J. A. et al. Progress on plague vaccine development. Appl Microbiol Biotechnol.91, 265–286 (2011). 10.1007/s00253-011-3380-6 [DOI] [PubMed] [Google Scholar]
- 46.Feodorova, V. A. & Motin, V. L. Plague vaccines: current developments and future perspectives. Emerg. Microbes Infect.1, e36 (2012). 10.1038/emi.2012.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenzweig, J. A. & Chopra, A. K. The future of plague vaccines: hopes raised by a surrogate, live-attenuated recombinant vaccine candidate. Expert Rev. Vaccines.11, 659–661 (2012). 10.1586/erv.12.34 [DOI] [PubMed] [Google Scholar]
- 48.Feodorova V. A. et al. Russian vaccines against especially dangerous bacterial pathogens. Emerg Microbes Infect. 310.1038/emi.2014.82. (2014). [DOI] [PMC free article] [PubMed]
- 49.Feodorova, V. A., Sayapina, L. V. & Motin, V. L. Assessment of live plague vaccine candidates. Methods Mol. Biol.1403, 487–498 (2016). 10.1007/978-1-4939-3387-7_27 [DOI] [PubMed] [Google Scholar]
- 50.Verma S. K. and Tuteja U. Plague vaccine development: Current research and future trends. Frontiers in Immunology7; 10.3389/fimmu.2016.00602 (2016). [DOI] [PMC free article] [PubMed]
- 51.Sun, W. & Singh, A. K. Plague vaccine: recent progress and prospects. NPJ Vaccines.4, 11 (2019). 10.1038/s41541-019-0105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenzweig, J. A., Hendrix, E. K. & Chopra, A. K. Plague vaccines: new developments in an ongoing search. Appl. Microbiol. Biotechnol.105, 4931–4941 (2021). 10.1007/s00253-021-11389-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The Australian Immunisation Handbook, 8th edition, NHMRC, Australia. Australian Immunisation Handbook, 8th Edition Part 3: Vaccines Listed by Disease (wordpress.com) (2003).
- 54.Sagiyev, Z. et al. Human response to live plague vaccine EV, Almaty region, Kazakhstan, 2014–2015. PloS ONE14, e0218366 (2019). 10.1371/journal.pone.0218366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feodorova, V. A. & Corbel, M. J. Prospects for new plague vaccines. Expert Rev. Vaccines.8, 1721–1738 (2009). 10.1586/erv.09.129 [DOI] [PubMed] [Google Scholar]
- 56.Kilgore, P. B. et al. Combinatorial viral vector-based and live attenuated vaccines without an adjuvant to generate broader immune responses to effectively combat pneumonic plague. mBio12, e03223–21 (2021). 10.1128/mBio.03223-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quenee, L. E. et al. Hereditary Hemochromatosis Restores the Virulence of Plague Vaccine Strains. J. Infect. Dis.206, 1050–1058 (2012). 10.1093/infdis/jis433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bacon, G. A. & Burrows, T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br. J. Exp. Pathol.37, 481–493 (1956). [PMC free article] [PubMed] [Google Scholar]
- 59.Williamson, E. D. et al. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol. Med Microbiol.12, 223–230 (1995). 10.1111/j.1574-695X.1995.tb00196.x [DOI] [PubMed] [Google Scholar]
- 60.Heath, D. G. et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine16, 1131–1137 (1998). 10.1016/S0264-410X(98)80110-2 [DOI] [PubMed] [Google Scholar]
- 61.Williamson, E. D. et al. Kinetics of the immune response to the (F1 + V) vaccine in models of bubonic and pneumonic plague. Vaccine25, 1142–1148 (2007). 10.1016/j.vaccine.2006.09.052 [DOI] [PubMed] [Google Scholar]
- 62.Quenee, L. E. et al. Plague in Guinea pigs and its prevention by subunit vaccines. Am. J. Pathol.178, 1689–1700 (2011). 10.1016/j.ajpath.2010.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demeure, C. E. et al. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun.20, 357–370 (2019). 10.1038/s41435-019-0065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erova T. E. et al. Evaluation of Protective Potential of Yersinia pestis Outer Membrane Protein Antigens as Possible Candidates for a New-Generation Recombinant Plague Vaccine. Clin. Vaccine Immunol.2010.1128/CVI.00597-12 (2013). [DOI] [PMC free article] [PubMed]
- 65.Matson, J. S. et al. Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol.5, 38–55 (2005). 10.1186/1471-2180-5-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivanov M. I. et al.. Vaccination of Mice with a Yop Translocon Complex Elicits Antibodies That Are Protective against Infection with F1−Yersinia pestis. Infect Immun7610.1128/iai.00189-08 (2008). [DOI] [PMC free article] [PubMed]
- 67.Swietncki, W., Powell, B. S. & Goodwin, J. Yersinia pestis Yop secretion protein F: purification, characterization and protective efficacy against bubonic plague. Protein Expr. Purif.42, 166–172 (2005). 10.1016/j.pep.2005.02.016 [DOI] [PubMed] [Google Scholar]
- 68.Williamson, E. D. et al. Recombinant (F1 + V) vaccine protects cynomolgus macaques against pneumonic plague. Vaccine29, 4771–4777 (2011). 10.1016/j.vaccine.2011.04.084 [DOI] [PubMed] [Google Scholar]
- 69.Fellows, P. et al. Characterization of a cynomolgus macaque model of pneumonic plague for evaluation of vaccine efficacy. Clin. Vaccin. Immunol.22, 1070–1078 (2015). 10.1128/CVI.00290-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fellows, P. et al. Protection in mice passively immunized with serum from cynomolgus macaques and humans vaccinated with recombinant plague vaccine (rF1V). Vaccine28, 7748–7756 (2010). 10.1016/j.vaccine.2010.09.062 [DOI] [PubMed] [Google Scholar]
- 71.Quenee, L. E. et al. Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine29, 6572–6583 (2011). 10.1016/j.vaccine.2011.06.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aftalion, M. et al. Rapid induction of protective immunity against pneumonic plague by Yersinia pestis Polymeric F1 and LcrV Antigens. Vaccines11, 581 (2023). 10.3390/vaccines11030581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones, T. et al. Intranasal Protollin/F1-V vaccine elicits respiratory and serum antibody responses and protects mice against lethal aerosolized plague infection. Vaccine6, 1625–1632 (2006). 10.1016/j.vaccine.2005.09.052 [DOI] [PubMed] [Google Scholar]
- 74.Honko, A. N. et al. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun.74, 1113–1120 (2006). 10.1128/IAI.74.2.1113-1120.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mizel, S. B. et al. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin. Vaccin. Immunol.16, 21–28 (2009). 10.1128/CVI.00333-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner, D. A. et al. Single-dose combination nanovaccine induces both rapid and long-lived protection against pneumonic plague. Acta Biomater.100, 326–337 (2019). 10.1016/j.actbio.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang, X. O. et al. Manganese-based nanoparticle vaccine for combating fatal bacterial pneumonia. Adv. Mater.35, 2304514 (2023). 10.1002/adma.202304514 [DOI] [PubMed] [Google Scholar]
- 78.Dentovskaya, S. V. et al. Peptidoglycan-Free Bacterial Ghosts Confer Enhanced Protection against Yersinia pestis Infection. Vaccines10, 51 (2022). 10.3390/vaccines10010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson, G. W. J. et al. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun.64, 4580–4585 (1996). 10.1128/iai.64.11.4580-4585.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carvalho, A. et al. Use of Bioengineered Human Commensal Gut Bacteria-Derived Microvesicles for Mucosal Plague Vaccine Delivery and Immunization. Clin. Exp. Immunol.196, 287–304 (2019). 10.1111/cei.13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Byvalov, A. A., Konyshev, I. V., Uversky, V. N., Dentovskaya, S. V. & Anisimov, A. P. Yersinia Outer Membrane Vesicles as Potential Vaccine Candidates in Protecting against Plague. Biomolecules10, 1694 (2020). 10.3390/biom10121694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore, B. D. et al. Predictors of survival after vaccination in a pneumonic plague model. Vaccines10, 145 (2022). 10.3390/vaccines10020145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levy, Y. et al. Targeting of the Yersinia pestis F1 capsular antigen by innate-like B1b cells mediates a rapid protective response against bubonic plague. NPJ Vaccines3, 52 (2018). 10.1038/s41541-018-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goncharova, A. et al. Assessment of the effect of using immunomodulatory drugs for emergency prevention of experimental plague caused by a virulent strain of the main subspecies Yersinia pestis. J. Microbiol., Epidemiol. Immunobiol.101, 2024 (2024). 10.36233/0372-9311-415 [DOI] [Google Scholar]
- 85.Galloway, D. R. et al. Co-formulation of the rF1V plague vaccine with depot-formulated cytokines enhances immunogenicity and efficacy to elicit protective responses against aerosol challenge in mice. Front. Immunol.15, 1277526 (2024). 10.3389/fimmu.2024.1277526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marketing authorisation from the State Research Centre for Applied Microbiology amnd Biotechnology of the Federal Service for the Supervision of Consumer Rights and Protection (Russia); Registration LP-004808 Microencapsulated molecular plague vaccine (PMMM, 2024) https://www.rlsnet.ru/regdoc/vakcina-cumnaya-molekulyarnaya-mikroinkapsulirovannaya-vcmm-lp-004808-74621.
- 87.Goncharova, A. et al. Experimental Evaluation of Application of the Vaccine Strain Yersinia pestis EV NIIEG in Combination with Immune-Modulators. Problems of Particularly Dangerous Infections. 71-77. 10.21055/0370-1069-2020-2-71-77. (2020).
- 88.Tiner, B. L. et al. Immunisation of two rodent species with new live-attenuated mutants of Yersinia pestis CO92 induces protective long-term humoral- and cell-mediated immunity against pneumonic plague. NPJ Vaccines.1, 16020 (2016). 10.1038/npjvaccines.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tiner, B. L. et al. Combinational deletion of three membrane protein-encoding genes highly attenuates yersinia pestis while retaining immunogenicity in a mouse model of pneumonic plague. Infect. Immun.83, 1318–1338 (2015A). 10.1128/IAI.02778-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tiner, B. L. et al. Intramuscular Immunization of Mice with a Live-Attenuated Triple Mutant of Yersinia pestis CO92 Induces Robust Humoral and Cell-Mediated Immunity To Completely Protect Animals against Pneumonic Plague. Clin. Vaccin. Immunol.22, 1255–1268 (2015B). 10.1128/CVI.00499-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Lier, C. J. et al. Deletion of Braun lipoprotein and plasminogen-activating protease-encoding genes attenuates Yersinia pestis in mouse models of bubonic and pneumonic plague. Infect. Immun.82, 2485–2503 (2014). 10.1128/IAI.01595-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Junxia F. et al. Construction of a Live-Attenuated Vaccine Strain of Yersinia pestis EV76-B-SHUΔpla and Evaluation of Its Protection Efficacy in a Mouse Model by Aerosolized Intratracheal Inoculation.Cell. Infect. Microbiol.,1010.3389/fcimb.2020.00473 (2020). [DOI] [PMC free article] [PubMed]
- 93.Cote, C. K. et al. Protection Elicited by Attenuated Live Yersinia pestis Vaccine Strains against Lethal Infection with Virulent Y. pestis. Vaccines9, 161 (2021). 10.3390/vaccines9020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.WHO R&D blueprint: Plague vaccines workshop https://www.who.int/blueprint/what/norms-standards/Plague_vaccines_workshop-23-april-2018/en/ (2018).
- 95.Demeure, C. et al. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination and diagnostics. Microbes Infect.21, 202–212 (2019). 10.1016/j.micinf.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 96.Dentovskaya, S. V. et al. Molecular bases of vaccine-prevention of plague. Mol. Genet. Microbiol. Virol.28, 87–98 (2013). 10.3103/S089141681303004X [DOI] [Google Scholar]
- 97.Andersson, J. A. et al. Identification of New Virulence Factors and Vaccine Candidates for Yersinia pestis. Front Cell Infect. Microbiol.7, 448 (2017). 10.3389/fcimb.2017.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang, X., Zhang, X., Zhou, D. & Yang, R. Live-attenuated Yersinia pestis vaccines. Expert Rev. Vaccines.12, 677–686 (2013). 10.1586/erv.13.42 [DOI] [PubMed] [Google Scholar]
- 99.Humphreys, I. R. & Sebastian, S. Novel viral vectors in infectious diseases. Immunology153, 1–9 (2018). 10.1111/imm.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang, S. et al. Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases. Sig Transduct. Target Ther.8, 149 (2023). 10.1038/s41392-023-01408-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Derbise, A. et al. An encapsulated Yersinia pseudotuberculosis is a highly efficient vaccine against pneumonic plague. PLoS Negl. Trop. Dis.6, e1528 (2012). 10.1371/journal.pntd.0001528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Derbise A., et al. Complete Protection against Pneumonic and Bubonic Plague after a Single Oral Vaccination. PLoS Negl Trop Dis. 1610.1371/journal.pntd.0004162 (2015). [DOI] [PMC free article] [PubMed]
- 103.Demeure, C. E. et al. Humoral and cellular immune correlates of protection against bubonic plague by a live Yersinia pseudotuberculosis vaccine. Vaccine3, 123–129 (2019). 10.1016/j.vaccine.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 104.Derbise, A. et al. Subcutaneous vaccination with a live attenuated Yersinia pseudotuberculosis plague vaccine. Vaccine38, 1888–1892 (2020). 10.1016/j.vaccine.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 105.Wang, X. et al. Remodeling Yersinia pseudotuberculosis to generate a highly immunogenic outer membrane vesicle vaccine against pneumonic plague. PNAS119, e2109667119 (2022). 10.1073/pnas.2109667119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morton, M. et al. A Salmonella enterica serovar Typhi vaccine expressing Yersinia pestis F1 antigen on its surface provides protection against plague in mice. Vaccine22, 2524–2532 (2004). 10.1016/j.vaccine.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 107.Garmory, H. S. et al. Antibiotic-free plasmid stabilization by operator-repressor titration for vaccine delivery by using live Salmonella enterica Serovar typhimurium. Infect. Immun.73, 2005–2011 (2005). 10.1128/IAI.73.4.2005-2011.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leckenby, M. W. et al. Enhanced vaccine antigen delivery by Salmonella with antibiotic free Operator Repressor Titration-based plasmid stabilisation compared to chromosomal integration. Microb. Pathog.46, 201–206 (2009). 10.1016/j.micpath.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 109.Sanapala, S. et al. Multiple antigens of Yersinia pestis delivered by live recombinant attenuated Salmonella vaccine strains elicit protective immunity against plague. Vaccine5, 2410–2416 (2016). 10.1016/j.vaccine.2016.03.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sha, J. et al. The two murine lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect. Immun.72, 3987–4003 (2004). 10.1128/IAI.72.7.3987-4003.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qingmei, J. et al. Single vector platform vaccine protects against lethal respiratory challenge with Tier 1 select agents of anthrax, plague, and tularemia. Sci. Rep.8, 7009 (2018). 10.1038/s41598-018-24581-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tao, P. et al. A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio9, e01926–18 (2018). 10.1128/mBio.01926-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kilgore, P. B. et al. A new generation needle- and adjuvant-free trivalent plague vaccine utilizing adenovirus-5 nanoparticle platform. Npj vaccines6, 21 (2021). 10.1038/s41541-020-00275-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sha, J. et al. A Replication-Defective Human type 5 adenovirus-based trivalent vaccine confers complete protection against plague in mice and nonhuman primates. Clin. Vaccin. Immunol.23, 586–600 (2016). 10.1128/CVI.00150-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiuchiolo, M. J. et al. Protective immunity against respiratory tract challenge with Yersinia pestis in mice immunized with an adenovirus-based vaccine vector expressing V antigen. J. Infect. Dis.194, 1249–1257 (2006). 10.1086/507644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boyer, J. L. et al. Protective immunity against a lethal respiratory Yersinia pestis challenge induced by V antigen or the F1 capsular antigen incorporated into adenovirus capsid. Hum. Gene Ther.21, 891–901 (2010). 10.1089/hum.2009.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Folegatti, P. M. et al. Benefit-Risk Assessment of Vaccines by Technology Working Group BRAVATO, ex-V3SWG). Vaccines based on the replication-deficient simian adenoviral vector ChAdOx1: Standardized template with key considerations fora risk/benefit assessment. Vaccine40, 5248–5262 (2022). 10.1016/j.vaccine.2022.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.WHO Landscape of plague vaccine candidates (2023).
- 119.Albrecht, M. T. et al. Immunogenicity and efficacy of an anthrax/plague DNA fusion vaccine in a mouse model. FEMS Immunol. Med. Microbiol.65, 505–509 (2012). 10.1111/j.1574-695X.2012.00974.x [DOI] [PubMed] [Google Scholar]
- 120.Kon E. et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci Adv. 910.1126/sciadv.adg1036 (2023). [DOI] [PMC free article] [PubMed]
- 121.Shattock, R. J. et al. A self-amplifying RNA vaccine provides protection in a murine model of bubonic plague. Front. Microbiol.14, 1247041 (2023). 10.3389/fmicb.2023.1247041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang, G. et al. mRNA vaccines in disease prevention and treatment. Sig Transduct. Target Ther.8, 365 (2023). 10.1038/s41392-023-01579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee, G. H. & Lim, S. G. CpG-Adjuvanted Hepatitis B Vaccine (HEPLISAV-B®) Update. Expert Rev. Vaccines.20, 487–495 (2021). 10.1080/14760584.2021.1908133 [DOI] [PubMed] [Google Scholar]
- 124.Hartley, L., Harold, S. & Hawe, E. The efficacy, safety and immunogenicity of plague vaccines: a systemic literature review. Curr. Res. Immunol.4, 100072 (2023). 10.1016/j.crimmu.2023.100072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.21CFR 601.90-95. Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible Fed. Register67, 37988-37998 (2002) [PubMed]
- 126.Williamson, E. D. et al. Recent advances in predictive models and correlates of protection in testing biodefence vaccines. Expert Rev. Vaccines9, 527–537 (2010). 10.1586/erv.10.22 [DOI] [PubMed] [Google Scholar]
- 127.European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/ebola (2024).
- 128.Mogil, J. S., Philip, V. M., Tuttle, A. H. & Chesler, E. J. Comparing phenotypic variation between inbred and outbred mice. Nat. Methods15, 994–996 (2018). 10.1038/s41592-018-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Majumder, S. et al. Protection Induced by Oral Vaccination with a Recombinant Yersinia pseudotuberculosis Delivering Yersinia pestis LcrV and F1 Antigens in Mice and Rats against Pneumonic Plague. Infect. Immu.90, e0016522 (2022). 10.1128/iai.00165-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chichester, J. A. et al. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine27, 3471–3474 (2009). 10.1016/j.vaccine.2009.01.050 [DOI] [PubMed] [Google Scholar]
- 131.Smiley, S. T. Immune defense against pneumonic plague. Immunol. Rev.22, 256–271 (2008). 10.1111/j.1600-065X.2008.00674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williamson, E. D. & Oyston, P. C. F. in Plotkins Vaccines (8th edition) (Ed. Orenstein, W. A., Offit, P. A., Edwards, K. M. & Plotkin, S. A.) Chapter 46 (Elsevier, 2023).
- 133.Parent, M. A. et al. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun.74, 3381–3386 (2006). 10.1128/IAI.00185-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elvin, S. J. & Williamson, E. D. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Micro. Pathog.3, 177–184 (2004). 10.1016/j.micpath.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 135.Levy, Y. et al. T cells play an essential role in anti-F1 mediated rapid protection against bubonic plague. Vaccine29, 6866–6873 (2011). 10.1016/j.vaccine.2011.07.059 [DOI] [PubMed] [Google Scholar]
- 136.Hamzabegovic, F. et al. Flagellin adjuvanted F1/V subunit plague vaccine induces T cell and functional antibody responses with unique gene signatures. NPJ Vaccines.5, 6 (2020). 10.1038/s41541-020-0156-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Amemiya, K. et al. Binding sites of anti-LcrV monoclonal antibodies are more critical than the avidities and affinities for passive protection against Yersinia pestis infection in a bubonic plague model. Antibodies9, 37 (2020). 10.3390/antib9030037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Andrianaivoarimanana, V. et al. Potential human immunotherapeutics for plague. Immunother. Adv.1, 1–8 (2021). 10.1093/immadv/ltab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Biryukov, S. S. et al. Functional assays to screen and select monoclonal antibodies that target Yersinia pestis. Hum. Vaccines Immunotherapeutics19, 2 (2023). 10.1080/21645515.2023.2216085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.International Standard Plague antiserum[Almond, Rajerison, Williamson, personal communication].
- 141.Raveros X. Target Product Profiles Plague vaccines WHO https://cdn.who.int/media/docs/default-source/documents/r-d-blueprint-meetings/global-consultation-on-plague-vaccines/3_ximena-riveros_plague-tpp.pdf?sfvrsn=edbe8135_3 (2023).
- 142.Rocke, T. E. et al. Effects of climate change on plague exposure pathways and resulting disease dynamics. DoD. Final Rep.16, RC01–RC012 (2020). [Google Scholar]
- 143.Xu, L. et al. Climate-driven marmot-plague dynamics in Mongolia and China. Sci. Rep.13, 11906 (2023). 10.1038/s41598-023-38966-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leal Filho, W. et al. A Review of Concepts, Definitions, and Bibliometrics. Int J. Environ. Res. Public Health19, 893 (2022). 10.3390/ijerph19020893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bartlow, A. W. et al. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet. Sci.6, 40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.WHO releases first data on global vaccine market since COVID-19 https://www.who.int/news/item/09-11-2022-who-releases-first-data-on-global-vaccine-market-since-covid-19.
- 147.WHO The Immunisation Agenda 2030 https://www.who.int/publications/i/item/9789240062726.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.