Abstract
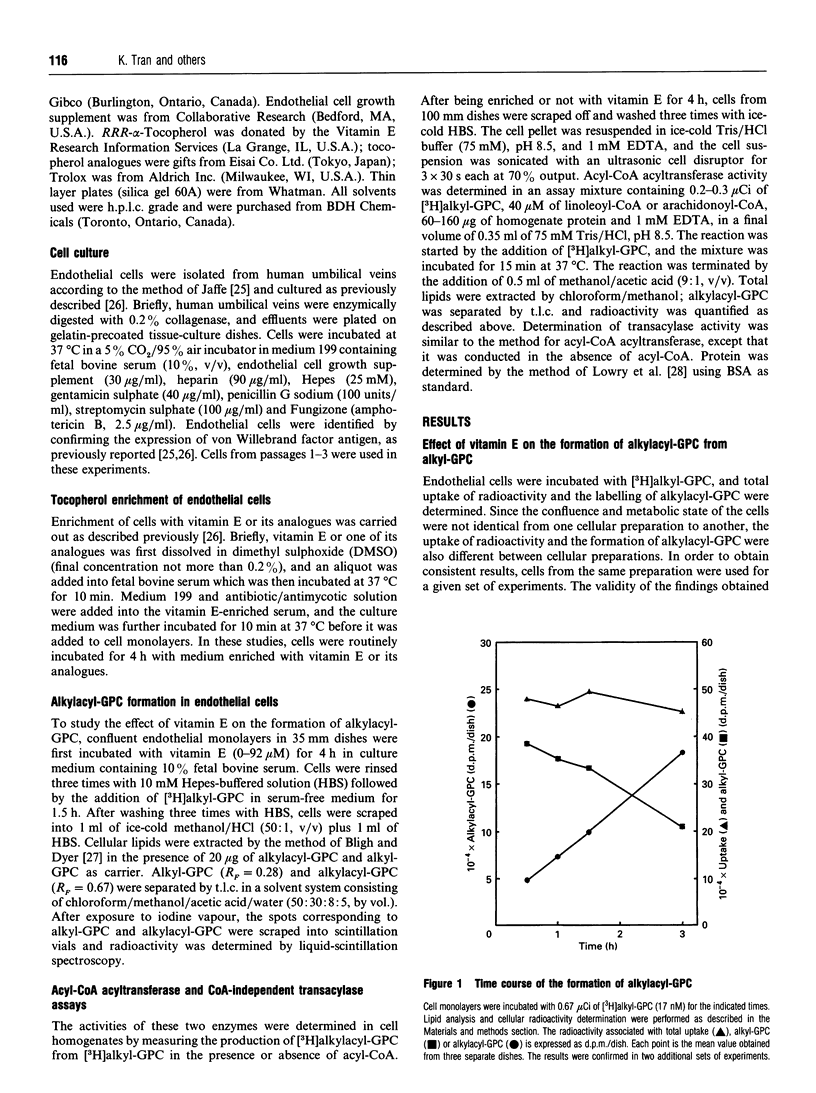
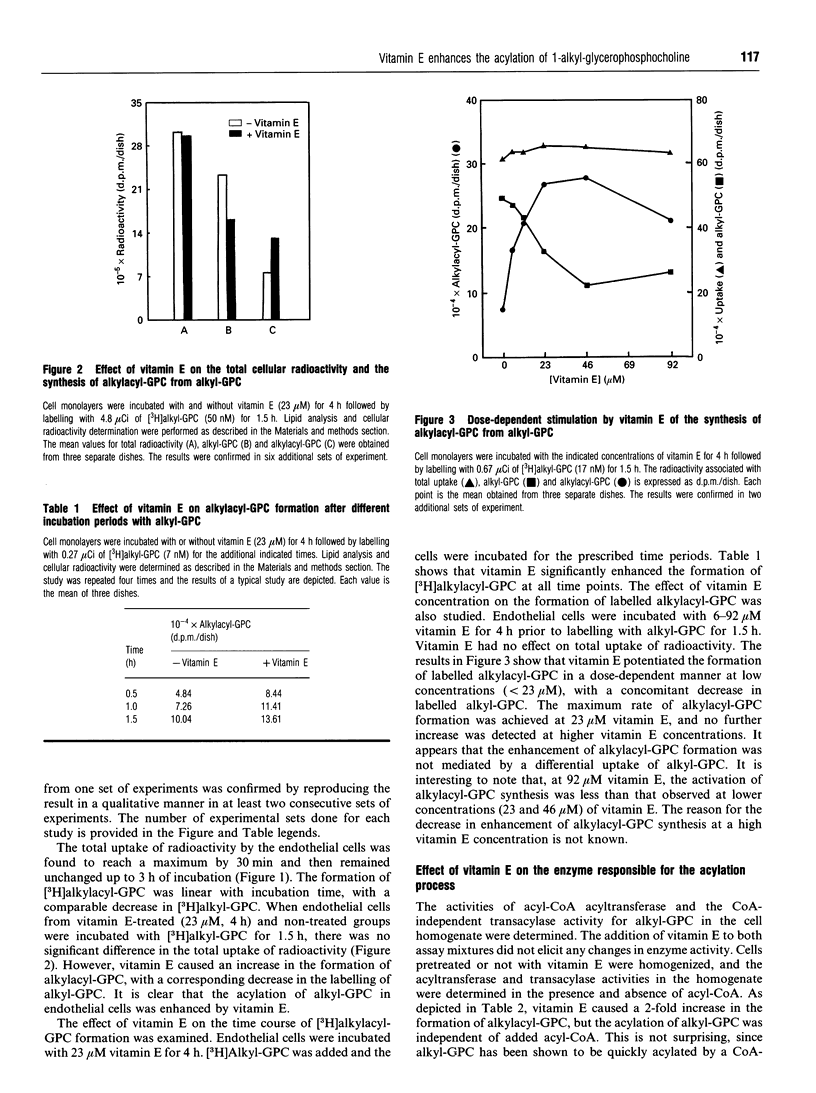
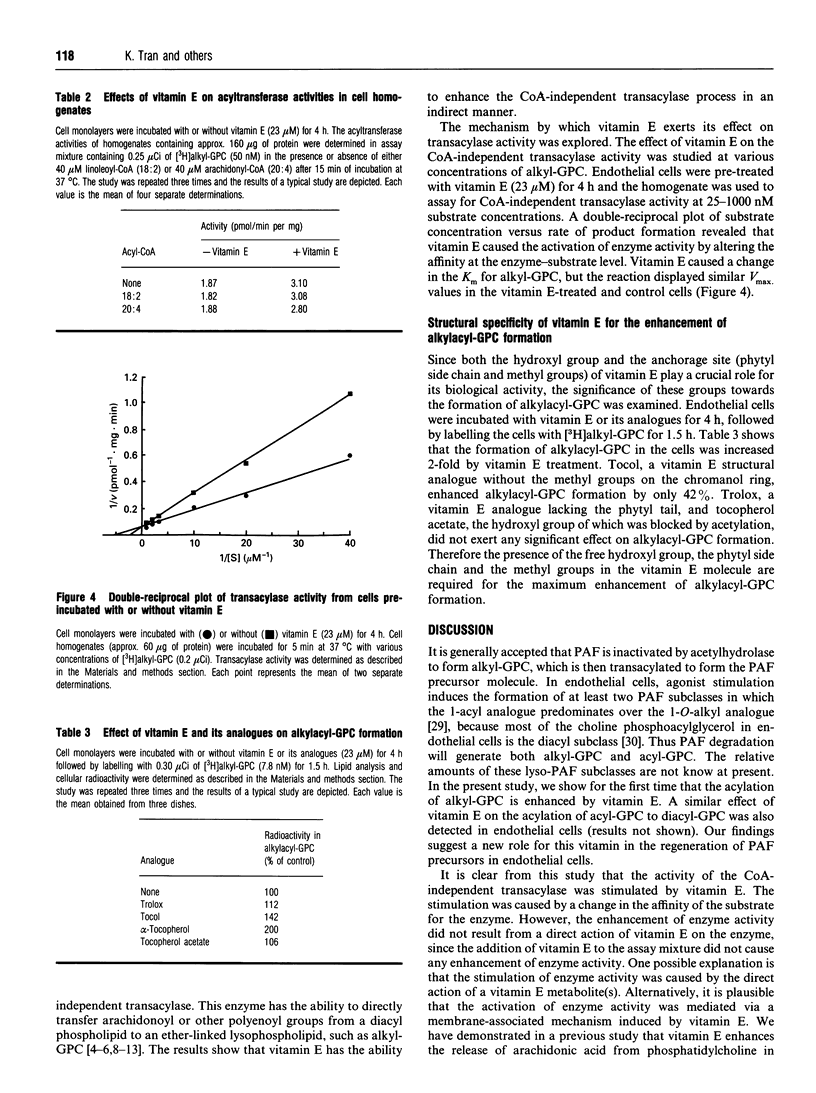
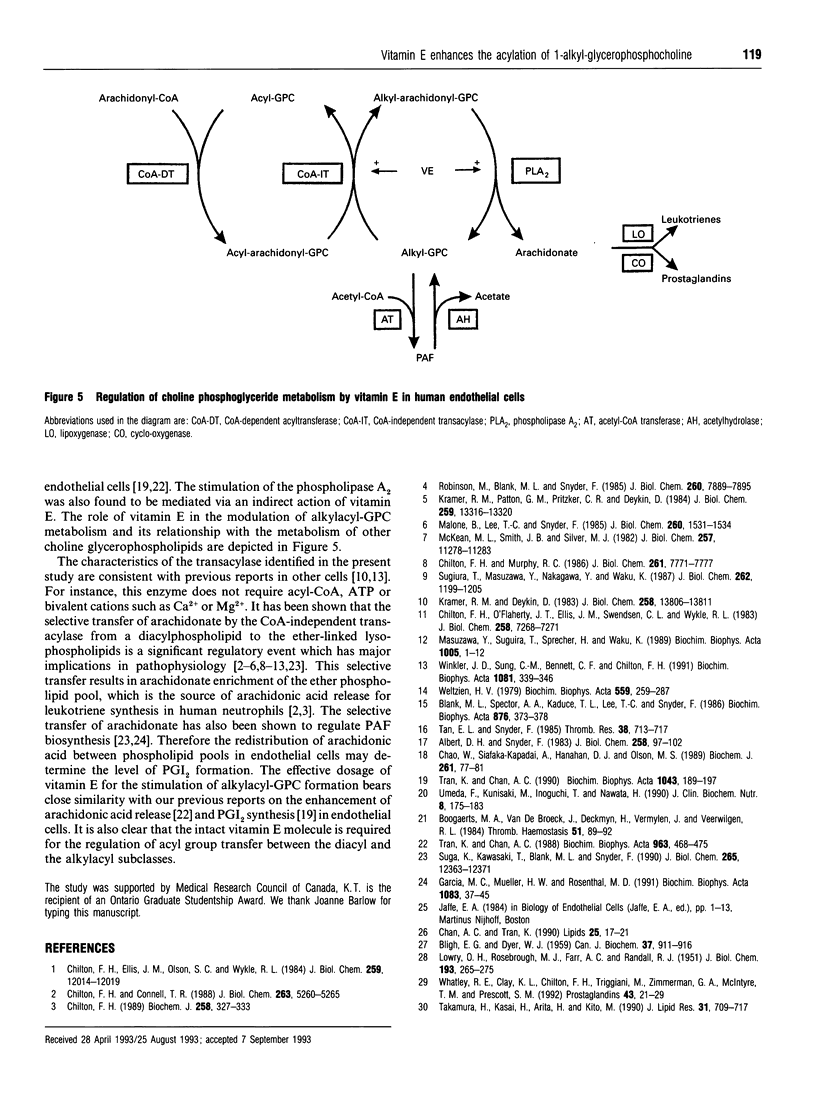
1-O-Alkyl-2-acyl-sn-glycero-3-phosphocholine (alkylacyl-GPC) is the precursor of platelet-activating factor. It is formed via the CoA-independent transacylase reaction, which transfers the polyenoyl acyl group from the sn-2 position of a diacyl phospholipid to the sn-2 position of 1-O-alkyl-sn-glycero-3-phosphocholine (alkyl-GPC). We have reported previously that vitamin E alters phospholipid turnover in the endothelial cells by increasing arachidonic acid release and prostacyclin synthesis. In the present study, the role of vitamin E in the formation of alkylacyl-GPC was investigated. Incubation of endothelial cells with vitamin E resulted in an increase in the formation of [3H]alkylacyl-GPC from [3H]alkyl-GPC. The effect of vitamin E was dose-dependent at concentrations below 23 microM. However, vitamin E did not have a direct effect on the transacylase activity. When endothelial cells were incubated with vitamin E, the CoA-independent transacylase activity in the cell homogenate was found to be enhanced. Kinetic analysis of the transacylase activity in the pre-incubated cells showed that the enhancement of enzyme activity was at the enzyme-substrate level. When endothelial cells were incubated with vitamin E analogues (Trolox, tocol and tocopherol acetate), only limited enhancement of the transacylation process was detected. It is clear that vitamin E enhanced the synthesis of alkylacyl-GPC from alkyl-GPC in a very specific manner by an indirect stimulation of the CoA-independent transacylase activity. The regulation by vitamin E of the formation of alkylacyl-GPC may mediate the transfer of arachidonate from the diacyl phospholipid pool into the ether-linked phospholipid pool.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Snyder F. Biosynthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine by rat alveolar macrophages. Phospholipase A2 and acetyltransferase activities during phagocytosis and ionophore stimulation. J Biol Chem. 1983 Jan 10;258(1):97–102. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Spector A. A., Kaduce T. L., Lee T. C., Snyder F. Metabolism of platelet activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) and 1-alkyl-2-acetyl-sn-glycerol by human endothelial cells. Biochim Biophys Acta. 1986 May 21;876(3):373–378. doi: 10.1016/0005-2760(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Boogaerts M. A., Van de Broeck J., Deckmyn H., Roelant C., Vermylen J., Verwilghen R. L. Protective effect of vitamin E on immune triggered, granulocyte mediated endothelial injury. Thromb Haemost. 1984 Feb 28;51(1):89–92. [PubMed] [Google Scholar]
- Chan A. C., Tran K. The uptake of (R,R,R)alpha-tocopherol by human endothelial cells in culture. Lipids. 1990 Jan;25(1):17–21. doi: 10.1007/BF02562422. [DOI] [PubMed] [Google Scholar]
- Chao W., Siafaka-Kapadai A., Hanahan D. J., Olson M. S. Metabolism of platelet-activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) and lyso-PAF (1-O-alkyl-2-lyso-sn-glycero-3-phosphocholine) by cultured rat Kupffer cells. Biochem J. 1989 Jul 1;261(1):77–81. doi: 10.1042/bj2610077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton F. H., Connell T. R. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J Biol Chem. 1988 Apr 15;263(11):5260–5265. [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Chilton F. H., Murphy R. C. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem. 1986 Jun 15;261(17):7771–7777. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Ellis J. M., Swendsen C. L., Wykle R. L. Selective acylation of lyso platelet activating factor by arachidonate in human neutrophils. J Biol Chem. 1983 Jun 25;258(12):7268–7271. [PubMed] [Google Scholar]
- Chilton F. H. Potential phospholipid source(s) of arachidonate used for the synthesis of leukotrienes by the human neutrophil. Biochem J. 1989 Mar 1;258(2):327–333. doi: 10.1042/bj2580327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. C., Mueller H. W., Rosenthal M. D. C20 polyunsaturated fatty acids and phorbol myristate acetate enhance agonist-stimulated synthesis of 1-radyl-2-acetyl-sn-glycero-3-phosphocholine in vascular endothelial cells. Biochim Biophys Acta. 1991 Apr 24;1083(1):37–45. doi: 10.1016/0005-2760(91)90122-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Deykin D. Arachidonoyl transacylase in human platelets. Coenzyme A-independent transfer of arachidonate from phosphatidylcholine to lysoplasmenylethanolamine. J Biol Chem. 1983 Nov 25;258(22):13806–13811. [PubMed] [Google Scholar]
- Kramer R. M., Patton G. M., Pritzker C. R., Deykin D. Metabolism of platelet-activating factor in human platelets. Transacylase-mediated synthesis of 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. J Biol Chem. 1984 Nov 10;259(21):13316–13320. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malone B., Lee T., Snyder F. Inactivation of platelet activating factor by rabbit platelets. Lyso-platelet activating factor as a key intermediate with phosphatidylcholine as the source of arachidonic acid in its conversion to a tetraenoic acylated product. J Biol Chem. 1985 Feb 10;260(3):1531–1534. [PubMed] [Google Scholar]
- Masuzawa Y., Sugiura T., Sprecher H., Waku K. Selective acyl transfer in the reacylation of brain glycerophospholipids. Comparison of three acylation systems for 1-alk-1'-enylglycero-3-phosphoethanolamine, 1-acylglycero-3-phosphoethanolamine and 1-acylglycero-3-phosphocholine in rat brain microsomes. Biochim Biophys Acta. 1989 Sep 11;1005(1):1–12. doi: 10.1016/0005-2760(89)90024-6. [DOI] [PubMed] [Google Scholar]
- McKean M. L., Smith J. B., Silver M. J. Phospholipid biosynthesis in human platelets. Formation of phosphatidylcholine from 1-acyl lysophosphatidylcholine by acyl-CoA:1-acyl-sn-glycero-3-phosphocholine acyltransferase. J Biol Chem. 1982 Oct 10;257(19):11278–11283. [PubMed] [Google Scholar]
- Robinson M., Blank M. L., Snyder F. Acylation of lysophospholipids by rabbit alveolar macrophages. Specificities of CoA-dependent and CoA-independent reactions. J Biol Chem. 1985 Jul 5;260(13):7889–7895. [PubMed] [Google Scholar]
- Suga K., Kawasaki T., Blank M. L., Snyder F. An arachidonoyl (polyenoic)-specific phospholipase A2 activity regulates the synthesis of platelet-activating factor in granulocytic HL-60 cells. J Biol Chem. 1990 Jul 25;265(21):12363–12371. [PubMed] [Google Scholar]
- Takamura H., Kasai H., Arita H., Kito M. Phospholipid molecular species in human umbilical artery and vein endothelial cells. J Lipid Res. 1990 Apr;31(4):709–717. [PubMed] [Google Scholar]
- Tan E. L., Snyder F. Metabolism of platelet activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) by capillary endothelial cells isolated from rat epididymal adipose tissue. Thromb Res. 1985 Jun 15;38(6):713–717. doi: 10.1016/0049-3848(85)90215-4. [DOI] [PubMed] [Google Scholar]
- Tran K., Chan A. C. Effect of vitamin E enrichment on arachidonic acid release and cellular phospholipids in cultured human endothelial cells. Biochim Biophys Acta. 1988 Dec 16;963(3):468–475. doi: 10.1016/0005-2760(88)90315-3. [DOI] [PubMed] [Google Scholar]
- Tran K., Chan A. C. R,R,R-alpha-tocopherol potentiates prostacyclin release in human endothelial cells. Evidence for structural specificity of the tocopherol molecule. Biochim Biophys Acta. 1990 Apr 2;1043(2):189–197. doi: 10.1016/0005-2760(90)90295-9. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Whatley R. E., Clay K. L., Chilton F. H., Triggiani M., Zimmerman G. A., McIntyre T. M., Prescott S. M. Relative amounts of 1-O-alkyl- and 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in stimulated endothelial cells. Prostaglandins. 1992 Jan;43(1):21–29. doi: 10.1016/0090-6980(92)90061-w. [DOI] [PubMed] [Google Scholar]
- Winkler J. D., Sung C. M., Bennett C. F., Chilton F. H. Characterization of CoA-independent transacylase activity in U937 cells. Biochim Biophys Acta. 1991 Feb 5;1081(3):339–346. doi: 10.1016/0005-2760(91)90291-o. [DOI] [PubMed] [Google Scholar]


