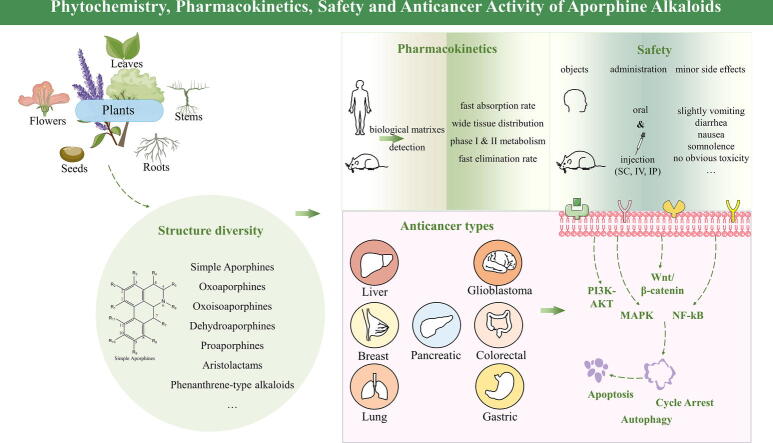
Graphical abstract
Keywords: Aporphine alkaloids, Anticancer, Phytochemistry, Pharmacokinetics, Application
Highlights:
-
•
The chemical structures and botanical diversity of aporphine alkaloids (AAs) are elucidated.
-
•
Pharmacokinetics and safety of AAs in animals and clinical trials are highlighted.
-
•
Some of AAs exhibit potent anticancer activities.
-
•
Treatment with AAs alone or a synergistic treatment with metal/clinical therapeutic drugs shows initial therapeutic promise.
-
•
AAs is the potential ingredient to develop the functional food containing plant-derived AA and the clinical application of AAs.
Abstract
Background
Cancer is the most common cause of death and is still a serious public health problem. Alkaloids, a class of bioactive compounds widely diffused in plants, especially Chinese herbs, are used as functional ingredients, precursors, and lead compounds in food and clinical applications. Among them, aporphine alkaloids (AAs), as an important class of isoquinoline alkaloids, exert a strong anticancer effect on multiple cancer types.
Aim of review
This review aims to comprehensively summarize the phytochemistry, pharmacokinetics, and bioavailability of seven subtypes of AAs and their derivatives from various plants and highlight their anticancer bioactivities and mechanisms of action.
Key Scientific Concepts of Review.
The chemical structures and botanical diversity of AAs are elucidated, and promising results are highlighted regarding the potent anticancer activities of AAs and their derivatives, contributing to their pharmacological benefits. This work provides a better understanding of AAs and combinational anticancer therapies involving them, thereby improving the development of functional food containing plant-derived AA and the clinical application of AAs.
Introduction
Cancer has become globally public health and economic problems caused by its serious morbidity and mortality. In 2022, approximately 4.8 million and 2.3 million new cancer cases, and 3.2 million and 0.6 million cancer deaths occurred in China and the USA, respectively [1]. Apart from the genetic risks, epidemiological studies showed that lifestyle, including smoking, excessive alcohol consumption, unhealthy dietary pattern, and physical inactivity, is associated with high cancer risk [2], [3]. As the field has evolved in cancer research and understanding in recent years, therapeutic options for cancer treatment include surgery, chemotherapy, and radiotherapy, which are accompanied by many side effects, such as incomplete removal of the tumor, metastasis development, recurrence, chronic pain and drug resistance. Researchers are now focusing on the use of natural phytochemicals, which show beneficial effects on cancer prevention and treatment with low toxicity [4], [5], [6].
Natural compounds are considered as promising strategies for cancer and inflammatory diseases [7], [8], [9]. The discovery of phytotherapy dates back to ancient Mesopotamia about 2,600 BCE. From 1980s, natural product drugs of the global approved antitumor drugs accounts for 33.5 % [7], [10]. Notably, alkaloids have attracted tremendous attention due to their wide clinical application. For example, Taxol/paclitaxel, as a diterpene alkaloids derived from the bark of the yew tree Taxus brevifolia Nutt., was approved as chemotherapy drug in 1993 for treatment of various cancers including breast, ovarian, pancreatic and prostate cancer [10], [11], [12]. Besides, natural alkaloids such as vinblastine and vincristine, and their derivatives vindesine and vinorelbine are anticancer drugs in clinic, showing bioactivities against neoplasms, chorio carcinoma hodgkins disease and non‑small cell bronchial carcinoma [10], [13].
Aporphine alkaloids (AAs, also called aporphinoids) are a class of isoquinoline alkaloids generally characterized by a tetracyclic aromatic basic N-containing skeleton (shown in Fig. 1) [14]. AAs are secondary metabolites found common throughout the plant kingdom, including Nymphaeaceae, Ranunculaceae and Annonaceae [15], [16], [17]. The applications of AAs in clinic dates back to the Qin and Han Dynasties, known as preventing fevers and insomnia with dietary herbal medicines such as lotus leaf, as recorded in “Shennong Bencao Jing” [18]. Actually, different active AAs has been identified and explored (shown in Table 1). AAs significantly inhibited the initiation, development and metastasis of tumor by regulating cellular functions and immune response, such as perturbation of cell cycle, programmed cell death and pro-inflammatory cytokines [2], [19], [20], [21]. AAs also play the key role in inhibiting the cancer cell proliferation through glycolytic pathway [22].
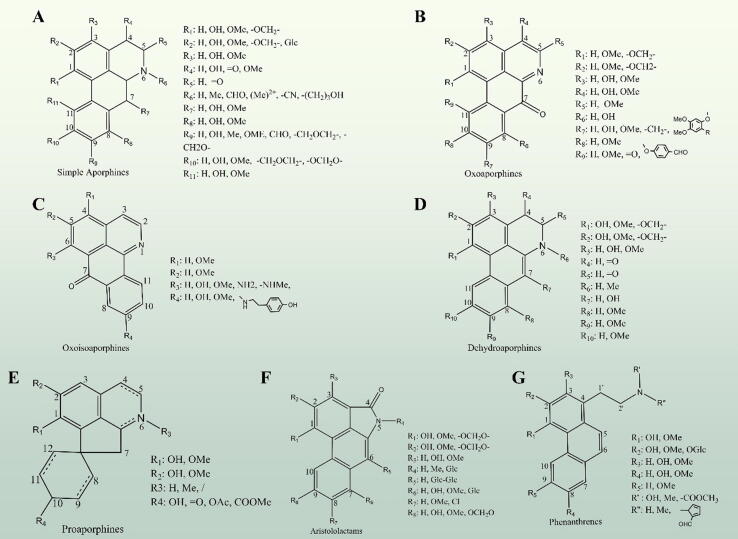
Fig. 1.
The basic skeleton of AAs, involving simple aporphines (A), oxoaporphines (B), oxoisoaporphines (C), dehydroaporphines (D), proaporphines (E), aristolactams (F), and phenanthrene (G) alkaloids.
Table 1.
Natural sources of AAs with anticancer activity.
| Botanical family | Plant name | Aporphine type | Compound name | Parts | Content (mg/kg) | Ref. |
|---|---|---|---|---|---|---|
| Nymphaeaceae | Nelumbo nucifera Gaertn (called lotus) | Simple aporphines | anonaine | leaves | 3.3 | [41] |
| asimilobine | 2.7 | |||||
| caaverine | 2.7 | |||||
| N-methylasimilobine | 10.7 | |||||
| nuciferine | 13.3 | |||||
| nornuciferine | 11.3 | |||||
| roemerine | 4.0 | |||||
| oxoaporphines | liriodenine | 2.7 | ||||
| lysicamine | 10.0 | |||||
| dehydroaporphines | 7-hydroxydehydronuciferine | 8.0 | ||||
| cepharadione B | 10.0 | |||||
| simple aporphines | nuciferine | flower buds | 42.2 | [34] | ||
| nornuciferine | 7.7 | |||||
| N-methylasimilobine | 17.5 | |||||
| asimilobine | 9.7 | |||||
| proaporphines | pronuciferine | 15.2 | ||||
| simple aporphines | nuciferine | leaves and flower buds | 74.8 ∼ 92.2; 148 ∼ 203.3 | [42] | ||
| N-nornuciferine | 2.6; 11.2 ∼ 134.4 | |||||
| N-methylasimilobine | 313.3; 6.6 ∼ 40 | |||||
| N-methylasimilobine N-oxide | 3.7; - | |||||
| nuciferine N-oxide | 24.6 ∼ 45.2; - | |||||
| asimilobine | 165.6; - | |||||
| (-)-lirinidine | 8.0; 3.3 | |||||
| oxoaporphines | lysicamine | 3.3 ∼ 46.4; 36.5 ∼ 102 | ||||
| proaporphines | pronuciferine | 9.2; 25.6 ∼ 56 | ||||
| dehydroaporphine | dehydronuciferine | 4.3; - | ||||
| 2-hydroxy-1-methoxy-6a,7-dehydroaporphine | 3.2; - | |||||
| Ranunculaceae | Thalictrum wangii | simple aporphines | (+)-8-(4′-formylphenoxy)glaucine | whole plants | 2.5 | [134] |
| (+)-8-(4′-hydroxymethylphenoxy)glaucine | 7.9 | |||||
| (+)-3-methoxy-8-(4′-formylphenoxy)glaucine | 2.2 | |||||
| oxoaporphines | 1,2,3,9,10-pentamethoxy-11-(4′-formylphenoxy)-7-oxoaporphine | 0.9 | ||||
| 1,2,9,10-tetramethoxy-11-(4′-formylphenoxy)-7-oxoaporphine | 0.5 | |||||
| proaporphine | 10-O-acetyl prodensiflorin A | 1.7 ∼ 2.3 | ||||
| Thalictrum foetidum | simple aporphines | (–)-9-(2′-methoxycarbonyl-5′, 6′-dimethoxyphenoxy)-1, 2, 3, 10-tetramethoxy aporphine | roots | 6.0 | [93] | |
| (–)-2′-methoxycarbonyl thaliadin | 2.8 | |||||
| (–)-9-(2′-methoxyethyl-5′, 6′-dimethoxyphenoxy)-1, 2, 3, 10-tetramethoxy aporphine | 0.3 | |||||
| (–)-3-methoxy hydroxyhernandalinol | 0.2 | |||||
| thaliadine | 1.1 | |||||
| oxoaporphines | 9-(2′-formyl-5′, 6′-dimethoxyphenoxy)-1, 2, 3, 10-tetramethoxy oxoaporphine | 0.4 | ||||
| 3-methoxy-2′-formyl oxohernandalin | 1.9 | |||||
| dehydroaporphine | 9-(2′-formyl-5′, 6′-dimethoxyphenoxy)-1, 2, 3, 10-tetramethoxy dehydroaporphine | 0.1 | ||||
| 3-methoxydehydrohernandaline | 0.2 | |||||
| Thalictrum cultratum | dimeric aporphine alkaloids | thalicultratine | roots | 0.4 ∼ 17.5 | [36] | |
| thalifaronine | 2.7 | |||||
| thalifaberine | 5.2 | |||||
| thalifabatine | 5.6 | |||||
| dehydrothalifaberine | 2.1 | |||||
| thalibealine | 0.8 | |||||
| Annonaceae | Fissistigma glaucescens (Hance) Merr | simple aporphines | (+)-nornuciferine | stems | 1.4 | [43] |
| norisocorydine | 1.5 | |||||
| laurotetanin | 1.8 | |||||
| oxoaporphines | Aporaloid | 1.2 ∼ 1.3 | ||||
| oxoxylopin | 15.3 | |||||
| micheline B | 2.3 | |||||
| dehydroaporphines | norcepharadione | 8.6 | ||||
| Enicosanthellum pulchrum (King) Heusden | oxoaporphines | liriodenine | roots | 80 | [25] | |
| Pseuduvaria trimera (Craib) | oxoaporphines | 8-hydroxyartabonatine C (6a,7-dehydro-1,4,5-trimethoxy-7-oxoaporphine) | leaves and twigs | 52.0 | [135] | |
| dehydroaporphines | ouregidione | 12.6 | ||||
| Xylopia laevigata | simple aporphines | (-)-Roemerine | stems | 2.7 | [101] | |
| (+)-Anonaine | 5.0 | |||||
| (+)-Glaucine | 0.7 | |||||
| (+)-Xylopine | 9.6 | |||||
| (+)-Norglaucine | 5.9 | |||||
| Asimilobine | 0.5 | |||||
| (+)-Norpurpureine | 3.1 | |||||
| (+)-N-methyllaurotetanine | 3.9 | |||||
| (+)-Norpredicentrine | 5.1 | |||||
| (+)-Calycinine | 1.1 | |||||
| (+)-Laurotetanine | 5.9 | |||||
| oxoaporphines | Lanuginosine | 3.6 | ||||
| Oxoglaucine | 5.9 | |||||
| Goniothalamus laoticus | simple aporphines | (−)-nordicentrine | flowers | 17.9 | [136] | |
| Lauraceae | Ocotea acutifolia (Nees) Mez. | simple aporphines | (+)-6S-ocoteine N-oxide | leaves and trunk barks | 50.0 | [137] |
| norocoxylonine | 13.0 | |||||
| (+)-6S-dicentrine N-oxide | 10.0 | |||||
| (+)-ocoteine | 42.0 | |||||
| (+)-ocoxylonine | 14.0 | |||||
| O-methylcassyfiline | 14.0 | |||||
| (+)-dicentrine | 20.0 | |||||
| leucoxine | 14.0 | |||||
| (+)-thalicsimidine | 8.0 | |||||
| (+)-isodomes ticine | 12.8 | |||||
| neolitsine | 10.6 | |||||
| oxoaporphines | halicminine | 30.0 | ||||
| Dehaasia longipedicellata | simple aporphines | boldine | barks | 5.0 | [138] | |
| norboldine | 5.0 | |||||
| papaveraceae | Dicranostigma leptopodum (Maxim) Fedde | simple aporphines | Isocorydione | whole plants | 1250 | [35] |
| Menispermaceae | Stephania dielsiana Y.C. Wu | oxoaporphines | oxostephanine | leaves | 1.2 | [127] |
| Stephania venosa (Blume) Spreng | simple aporphines | crebanine | leaves | 40.2 | [139] | |
| oxoaporphines | oxostephanine | 5.2 | ||||
| thailandine | 26.2 | |||||
| dehydroaporphine | 4,5-dioxo-dehydrocrebanine | 11.0 | ||||
| dehydrocrebanine (2) | 8.9 | |||||
| Stephania venosa (Blum) Spreng | simple aporphines | stephanine 3 | tubers | 37.5 | [140] | |
| crebanine 4 | 58.3 | |||||
| O-methylbulbocapnine 5 | 91.7 | |||||
| dehydroaporphine | dehydrocrebanine 1 | 55.8 | ||||
| Sinomemium acutum | simple aporphines | magnoflorine | stems and rhizomes | 388.8 | [40] | |
| oxoisoaporphines | dauriporphine | 25.6 | ||||
| bianfugecine | 0.8 | |||||
| dauriporphinoline | 5.6 | |||||
| menisporphine | 4.0 | |||||
| Formosan Cocculus orbiculatus | simple aporphines | (+)-laurelliptinhexadecan1-one | stems | 0.2 | [40] | |
| (+)-laurelliptinoctadecan-1-one | 0.2 | |||||
| Magnolia grandiflora L. | simple aporphines | magnoflorine | leaves | 3.3 | [39] | |
| anonaine | 2.2 | |||||
| oxoaporphines | lanuginosine | 3.9 | ||||
| liriodenine | 2.2 |
The relationship between the structure and bioactivities, especially anticancer effects, as well as the potential mechanisms of AAs are not well summarized because of the diversity of their chemical structures. This review summarizes the basic information of AAs, including their structures, botanical origins, and pharmacokinetics, as well as their anticancer activities and the mechanisms of action, to provide a theoretical basis in the application of AAs in clinical practice and food industry.
All available information on AAs was collected from scientific databases, including PubMed, Web of Science, ScienceDirect, Scopus, Springer, ACS, Wiley, and Google Scholar. The search terms used for this review included aporphine alkaloids, phytochemistry, pharmacokinetics, toxicity, anticancer, antitumor, and mechanism. The screening process of all papers primarily relied on reviewing their titles and abstracts and only articles in English were considered for inclusion in this review. Inclusion criteria were phytochemical property, clinical, in vivo, and in vitro researches on pharmacokinetics, toxicity, anticancer activities and mechanism. Exclusion criteria encompassed studies that focused on synthetic products or did not directly address the topic.
The phytochemistry of AAs
Structural diversity of AAs
AAs are mainly classified into seven subtypes based on various substitution and planar conformations, such as simple aporphines, oxoaporphines, oxoisoaporphines, dehydroaporphines, proaporphines, aristolactams, and phenanthrene-type alkaloids (Fig. 1). Simple aporphines are the most common subclasses of AAs, with a 5,6,6a,7-tetrahydro‐4H‐dibenzo[de,g]quinoline core, accompanied by various substitution groups at various positions (including hydroxyl, methoxy, methylenedioxy, and carbonyl group) [16]. Nitrogen is actively substituted with groups, including methyl or aldehyde, or cyanide (Fig. 1A). Among them, the N-methyl and N-(2-hydroxypropyl) substitution groups on the basic skeleton of simple aporphines, such as nuciferine and (+)-N-(2-hydroxypropyl) lindcarpine, are beneficial groups in terms of cytotoxicity on cancer cells [23]. Apomorphine, a semi-synthetic drug currently used to treat Parkinson’s disease, has one tertiary amine (aliphatic) and two aromatic hydroxyls, which is produced by reacting morphine with ZnCl2 or hydrochloric acid. Moreover, the structural modifications of simple aporphines have attracted researchers’ attention and new lead molecules containing a C10 nitrogen motif are synthesized and acting as the neurotransmitter serotonin receptors to resolve neuropsychiatric conditions [24].
Oxoaporphines and oxoisoaporphines are the derivatives of 1-azabenzanthrone that is composed of an aromatic quinoline ring attached to a tetralone unit. Unlike simple aporphines, oxoaporphines, and oxoisoaporphines have an aromatic N-containing ring and a carbonyl group at C7 position in the tetracyclic skeleton (Fig. 1B and C) [16]. According to studies on oxoaporphines activity, both liriodenine and dicentrinone have strong anti-protozoal and anticancer activities, suggesting that the oxo-group and 1, 2-methylenedioxy group are the key factors in the action of alkaloids [25]. Although the oxo-group highly improves the conjugation stability of oxo-AAs, a series of new derivatives, which contain a substitution of various amide groups at C4 and C5 position, are synthesized to enhance their cytotoxicity and inhibit bioactivity of topoisomerase [26]. Besides carbonyl substitution at the C7 position, hydroxyl or methoxy groups are sometimes present, known as 7‐oxygenated aporphines.
Different from the precedent subtypes, dehydroaporphines have a 5,6‐dihydro‐4H‐dibenzo [de, g] quinoline core with a double bond between C6a and C7 obtained through dehydrogenation, which provides sufficient nucleophilicity to dehydroaporphines for the introduction of carbon substituents (Fig. 1D) [16]. Interestingly, the study of the compounds didehydroglaucine and dehydrocrebanine revealed that the double bond at C6a and C7 plays a critical role in increasing cytotoxicity besides of the 1,2-methylenedioxy group [16]. Moreover, the substitution of the methyl (C1, C2), methoxy (C9, C10), and aldehyde group (nitrogen) at various positions of the dehydroaporphines forms an amidic dehydroapophine, which possesses wide pharmacological activity in vivo and in vitro.
Proaporphines are the biogenetic precursors to some specific AAs and are characterized by a new skeleton of an isoquinoline part fused to a pentacyclic ring and then sequentially connected to a benzyl part through a spiro carbon. Proaporphines generally show a substitution by hydroxyl, methoxy and methyl group at C1, C2, C10 position, and the methyl at N-atom occasionally forms double bonds through dehydrogenation, as shown in Fig. 1E by a dotted line. The naturally occurring proAA stepharine, which possesses antihypertensive activity and sedative effect, has 1,2-methoxy and carbonyl groups at C10 position, double bonds at C8 and C9, as well as C10 and C12 [27]. Nowadays, more natural proaporphines are consecutively extracted and identified from the plant kingdom, such as cissamaline, cissamanine, and cissamdine, isolated from the leaves and stems of Cissampelos capensis, consisting of three ring systems with various substitution groups at C1, C1a, C2, and nitrogen [28].
The basic skeleton of aristolactams (also called aristololactams) shows similarity to that of simple aporphines but with a unique structure in the N-containing ring (Fig. 1F). As a variety of aristolochic acid analogues, aristolactams have a phenanthrene chromophore and exert more potent bioactivities than aporphines due to the presence of the lactam group (typical –CONH- structure) at the N-containing ring [29]. It is well known that aristolactam alkaloids are not confined to the Aristolochiaceae, such as compounds identified in Fissistigma species (Annonaceae), including aristolactam AII, BII, and FII, which are substituted by a methoxy group at C1 and/or C2 position, and by a hydroxyl group at C2 and/or C9 position. Aristolactam alkaloids are further classified into four categories according to the difference in oxygenation pattern: dio- (such as 10-Amino-2,4-dimethoxy-phenanthrene1-carboxylic acid lactam 1), trio- (such as 10-Amino-1-carboxy-3,4-dimethoxy-6-hydroxyphenanthrene lactam N-β-D-glucopyranoside), tetrao- (such as aristolamide-N-hexoside), and pentao-xygenated (such as 7-Methoxyaristolactam IV) aristolactams. Most of these compounds possess antitumor activities against human cancer cells [29].
Fig. 1G shows the basic skeleton of phenanthrene-type alkaloids, which have an N-containing side chain with various substitutions. The phenanthrene-type alkaloids are generally replaced by hydroxyl and methoxy groups in the aromatic rings (known as electrophilic substitution reaction). The phenanthrene alkaloid atherosperminine is characterized by a methoxy substitution in C1 and C2 position, and two methyl groups in the N-atom, conferring the ability to strongly inhibit arachidonic acid-induced platelet aggregation [30]. The phenanthrene-type alkaloids originated from the same plants may have a similar structure, such as the compound atherosperminine, 2-hydroxyathersperminine, and noratherosperminine, which are isolated from the bark of Cryptocarya nigra and consistently presenting a substitution by methoxy groups at C1 and C2, although they are distinguished by the presence of a hydroxyl group at C3, and methyl groups at the N-atom [31].
However, the basic skeletons of AAs are not limited to those in Fig. 1A-G, for example, compound taspine that has a dilactonic, tertiary amine structure and dimethylaminoethyl side-chain, Besides carbonyl substitution at the C7 position, hydroxyl or methoxy groups are present, known as 7‐oxygenated aporphines [32]. Besides, Ali et al. obtained a set of dimeric AAs, which consist of two aporphinoid units through either an ether or less frequently a C–C bond, such as dehatriphine with isocorydine and N-methyl-laurotetanine units with an ether linkage [33]. Therefore, AAs exhibit structural diversity, which is helpful in developing clinical drugs to combat various cancers based on the original natural compounds (Fig. 2).
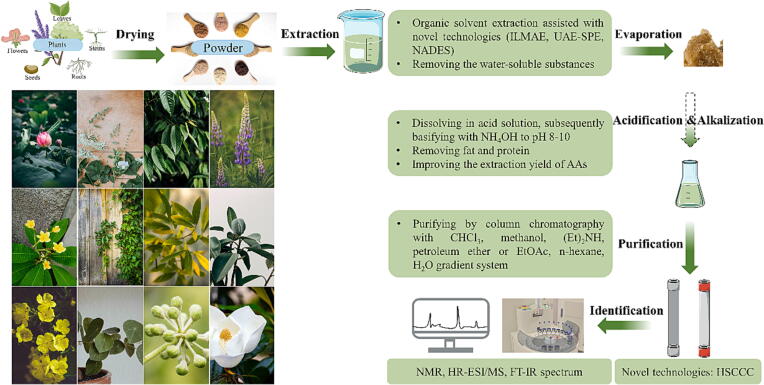
Fig. 2.
The plant species and schematic representation of the extraction, purification, and identification of AAs. ILMAE: ionic liquids-based microwave-assisted extraction; UAE-SPE: ultrasonic-assisted extraction-solid phase extraction; HSCCC: high-speed counter-current chromatography; NADES: natural deep eutectic solvents.
Botanical sources of AAs
AAs are primarily distributed within the families of Annonaceae, followed by Ranunculaceae, Nymphaeaceae, Lauraceae, papaveraceae, Menispermaceae and Magnoliaceae, which include various Chinese medicinal plants such as Nelumbo nucifera Gaertn [34], Thalictrum foetidum [35], and Thalictrum cultratum [36], [37]. Notably, AAs, such as nuciferine, pronuciferine, and dehydronuciferine, are potential characteristic ingredients in Nymphaeaceae (especially in lotus), while isocorydione is exclusively detected in Papaveraceae compared to other families. This indicates variations in compound distribution among different botanical families (Table 1). Furthermore, the content of compounds isolated from various plants or plant parts differs significantly. For instance, liriodenine, as an oxoAA, is more abundant in the roots of Enicosanthellum pulchrum (King) Heusden (80 mg/kg) than in other plants (<11 mg/kg) [25], [38], [39], and magnoflorine as a simple AA is richer in the stems and rhizomes of Sinomemium acutum (388.8 mg/kg) than in the leaves of Magnolia grandiflora L. (3.3 mg/kg) [39], [40]. Table 1 shows that the simple AA nuciferine is present in higher content in the flower buds (42.2 mg/kg) than that in the leaves (11.3 mg/kg) of N. nucifera Gaertn (called lotus), and the results are consistent with those of Nakamura et al. (148 ∼ 203.3 mg/kg in flower buds and 74.8 ∼ 92.2 mg/kg in leaves) [34], [41], [42]. To date, the structural compositions of new AAs from different plant parts have been widely reported, providing sufficient information for the identification and the discovery of new drugs, highlighting the biological diversity and pharmacological value of these natural products.
Extraction, purification, and identification of AAs
Considering the promising pharmacological effects of AAs from different plants, efficient extraction, purification, and identification of AAs are critical for research and application. Many strategies have been established not only classical approaches, but also green extraction techniques (Fig. 2).
The classical approach for AA extraction from the plants usually use organic solvents. Plant grounds into powder and macerated with organic solvents including methanol, ethanol, n-hexane or ethyl acetate. Macroporous adsorption resin is employed to enrich the crude extracts followed by dissolving in acidic solution, normally hydrochloric acid or acetic acid, to solubilize the alkaloids [43]. The extraction efficiency of AAs with organic solvent is impacted by parameters, such as solvent, temperature, pressure, and extraction time. Considering that AAs are a group of compounds sensitive to both light and heat, these factors present notable challenges during the extraction procedure [37]. Following extraction using acidic and basic solutions, the crude AAs extracts are subjected to silica gel column chromatography with gradient elution, commonly involving CH2Cl2-MeOH, CHCl3-MeOH, CH3COOCH3-MeOH, MeOH-H2O and petroleum ether-CH3COOCH3 gradient system [25]. The choice of eluents in chromatography is determined by the polarity of AAs. Identification is performed by using semi-preparative high-performance liquid chromatography (HPLC) in combination with spectroscopic assays, such as hydrogen nuclear magnetic resonance (H NMR), carbon-13 nuclear magnetic resonance (13C NMR), high-resolution electrospray ionization mass spectrometry (HR-ESI/MS), and fourier-transform infrared spectroscopy (FT-IR).
Moreover, the potential of green extraction techniques as an alternative to traditional extraction approaches has been extensively explored. One green extraction strategy is the use of non-toxic solvents, such as natural deep eutectic solvents (NDES) and supercritical carbon dioxide (Sc-CO2). NDES have gained increasing attention benefited from their simple preparation, low cost, biodegradability, sustainability, high solubilization power and acceptable pharmaceutical toxicity [44], [45], [46]. Menthol-camphor and menthol-thymol NDES mixtures yielded high amounts of chelidonine (35 %), berberine (76 %), and coptisine (180 %) compared with control extractants (water and methanol) in the extraction of Chelidonium majus plant [45]. Besides, the boldine extraction with proline-oxalic acid NDES mixture is eight-times more efficient than methanol in Peumus boldus Mol., (Monimiaceae) [46]. Choline chloride-fructose NDES mixture demonstrated a total alkaloidal extraction capacity of 2.43, 2.25 and 2.38 times than that of ethanol, methanol and water, respectively [47]. Sc-CO2 is commonly used in supercritical fluid extraction (SFE) due to its gas-like and liquid-like properties, selectivity in extracting specific compounds and can be easily removed by depressurization [48], [49]. In recent years, Sc-CO2 extraction is used to obtain the alkaloids from plants, such as Sophora moorcroftiana, N. tabacum leaves and Camellia sinensis (L.) Kuntze [50], [51], [52].
Other green extraction strategies are extraction combined with innovative technologies including ionic liquids-based microwave-assisted extraction (ILMAE), ultrasonic-assisted extraction-solid phase extraction (UAE-SPE), and pressurized liquid extraction (PLE) [52], [53], [54], [55]. Ionic liquids have been successfully applied in two-phase systems extraction due to their unique properties of negligible vapor pressure and volatility, high thermal stability and ease of handling. In the alkaloids extraction from lotus leaves, the application of ILMAE, specially 1-hexyl-3-methylimidazolium bromide as the ionic liquids, resulted in 26 % enhancement in efficiency and reduction in extraction time (from 2 h to 2 min) compared to the regular MAE technique [56]. UAE has been frequently assisted with various extraction solvents while UAE-SPE is successfully applied in production of high purity nuciferine with a purity over 98.5 % from lotus leaves [57]. Pretreatment such as concentration or re-dissolving before SPE is not required, and waste solvents from SPE can be used as UAE extractants without purification, resulting in significant energy and emission reductions [57]. Under high temperature and pressure conditions, the use of PLE effectively improves the compounds solvation and diffusion rates, simultaneously shorts the extraction time and reduce the solvent consumption [58]. PLE is generally targeted at polar compounds extraction and has been successfully used to extract alkaloids, such as caffeine and theobromine from cocoa shell [59].
Pharmacokinetics of AAs
The pharmacokinetics of AAs has attracted considerable scientific attention due to the multiple bioactivities of AAs identified in clinical practice and in vivo. Among them, the clinical pharmacokinetics of apomorphine and nuciferine have been well studied. The concentration of alkaloids in different biological matrices, including plasma, tissues, urine, and feces, has been determined, and the pharmacokinetic parameters, as well as the metabolic pathways are summarized in Fig. 3, Table 2, and Table 3.
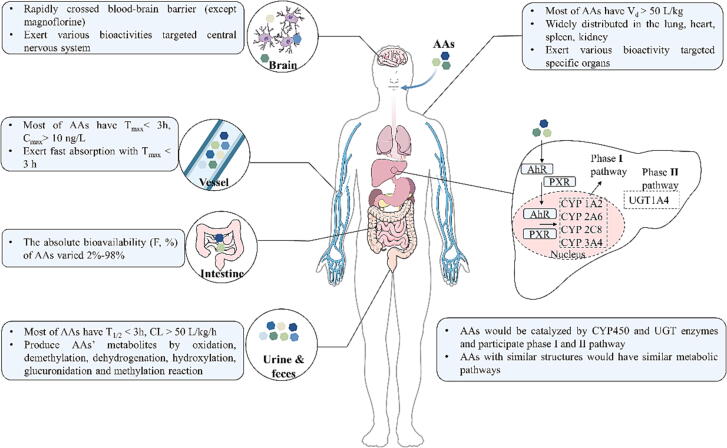
Fig. 3.
The absorption, distribution, metabolism, and excretion properties of AAs. AAs: aporphine alkaloids; AhR: the aryl hydrocarbon receptor; Cmax: maximum plasma concentration; CL: clearance rate; CYP: cytochrome P450; F: oral absolute bioavailability; PXR: the pregnane X receptor; Tmax: time to reach concentration peak; T1/2: terminal elimination half-life; UGT: uridine diphosphate glycosyltransferase; Vd: apparent volume of distribution.
Table 2.
Pharmacokinetic parameters of AA in various models.
| Name | Model | Dose | Treatment | Cmaxng/mL | Tmax (h) | T1/2 (h) | h*ng/mL | h*ng/mL | CLL/kg/h | VdL/kg | F/% | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| apomorphine | PD Patients (no smoking, 30–85 years old, BMI = 18–32 kg/m2) |
2–4 mg/kg | oral once daily for 1–5 days | 6.9–9.6 (2 mg/kg); 12–19.3 (3 mg/kg); 14.9–28.1 (4 mg/kg) | 1–2 | 0.6–0.7 | – | 2.3–2.6 (2 mg/kg); 4.5–7.1 (3 mg/kg); 5.7–8 (4 mg/kg) | – | – | – | [141] |
| 20–30 mg/kg | sublingual film | 4.3–11.2 | 0.6–0.8 | 1.1–1.2 | 7.2–22.8 | 7.2–22.8 | – | – | [60] | |||
| PD Patients (males or females ≥ 18 years old) | 3–5 mg/kg | subcutaneous injection | 6 (3 mg/kg); 10.3 (4 mg/kg); 12.4 (5 mg/kg) | 0.3–0.4 | 1.0–1.2 | 7.4–16 | 7.4–16 | – | – | 98 | ||
| 3–5 mg/kg | subcutaneous apomorphine prefilled injection pen | 7 (3 mg/kg); 11.5 (4 mg/kg); 16.8 (5 mg/kg) | 0.3–0.4 | 0.8–1.0 | 8–16.7 | 8–16.7 | – | – | 83 | |||
| nuciferine | Healthy volunteers (18–45 years old, BMI = 19–24 kg/m2) | 1.28–2.56 g Tangzhiqing tablet (nuciferine as its pharmacokinetics marker) | single-dose oral | 1.6 (1.28 g); 2.1 (1.92 g); 3.8 (2.56 g) | 1.8–2.0 | 1.3–1.6 | 5.3 (1.28 g); 7.4 (1.92 g); 13.3 (2.56 g) | 5.4 (1.28 g); 7.5 (1.92 g); 13.3 (2.56 g) | – | – | – | [61] |
| 1.92 g Tangzhiqing tablet | oral 3 times/day for 6 days | 2.4 | 1.6 | 1.9 | 9.3 | 10.5 | – | – | – | |||
| SD rat (male, 250 ± 10 g) | 28.8 mg/kg | Single dose oral | 317.6 | 3.5 | 6.18 | 2069 | 2142 | – | – | – | [64] | |
| SD rat (male, 250–300 g) | 0.2 mg/kg | IV | – | – | 0.6 | 119 | 119 | 1.8 | 1.5 | – | [62] | |
| 2–10 mg/kg | single dose oral | 13.5 (2 mg/kg); 63.4 (5 mg/kg); 103 (10 mg/kg) | 0.5–0.9 | 0.8–1.5 | 41.7 (2 mg/kg); 118.2 (5 mg/kg); 207.1 (10 mg/kg) | 41.7 (2 mg/kg); 118.2 (5 mg/kg); 207.5 (10 mg/kg) | 52.5–58.6 | 57.3–112.4 | 3.8–4.2 | |||
| 0.2 mg/kg | IV | – | – | 6.6 | 2022.8 | 2026.3 | 0.3 | 0.9 | 1.9 | [63] | ||
| 10 mg/kg | single dose oral | 45.3 | 1 | 6.5 | 280.6 | 308.2 | 64.9 | 616.2 | ||||
| SD rat (male, 200 ± 20 g) | 4.5 mg/kg | IV | – | 0 | 2.09 | 2090 | 2130 | 2.2 | 9.5 | 58 | [66] | |
| 22.5 mg/kg | single dose oral | 1710 | 0.9 | 2.48 | 6130 | 6200 | 2.2 | 7.9 | ||||
| N-nuciferine | SD rat (male, 200 ± 20 g) | 2 mg/kg | IV | – | 0 | 3.84 | 7400 | 8500 | 2.6 | 15.2 | 80 | [66] |
| 10 mg/kg | single dose oral | 570 | 1.65 | 2.94 | 3320 | 3400 | 2.4 | 10.3 | ||||
| O-nornuciferine | SD rat (male, 250 ± 10 g) | 9.36 mg/kg | single dose oral with lotus extract | 42.1 | 2.6 | 6.67 | 319.8 | 335.6 | – | – | – | [64] |
| pronuciferine | SD rat (male, 250 ± 10 g) | 9.41 mg/kg | single dose oral with lotus extract | 210 | 4.9 | 4.44 | 2031 | 2096 | – | – | – | [64] |
| liriodenine | SD rat (male, 250 ± 10 g) | 3.6 mg/kg | single dose oral with lotus extract | 54.7 | 5.7 | 3.77 | 415.1 | 429 | – | – | – | [64] |
| magnoflorine | SD rat (male, 200 ± 20 g) | 5 mg/kg | IV | 2453.4 | – | 1.5 | 2430 | 2450.4 | 2.1 | 4.5 | 23 | [72] |
| 15 mg/kg | single dose oral | 181.5 | – | 13.2 | 1645.6 | 2384.9 | 8 | 122.9 | ||||
| 10 mg/kg | IV | – | – | 3.31 | 143.3 | 162.9 | 0.1 | – | – | [142] | ||
| 10 mg/kg | IV | 3948 | – | 1.27 | 2510 | 2517 | 4.1 | 7.5 | – | [68] | ||
| 15 mg/kg | single dose oral | 81.7 | 1.13 | 1.3 | 321.1 | 334.3 | 50.6 | 102.6 | 8.9 | |||
| 30 mg/kg | single dose oral | 145.1 | 1.21 | 1.7 | 534.3 | 548 | 57.1 | 133.9 | 7.3 | |||
| 60 mg/kg | single dose oral | 260 | 1.04 | 1.5 | 787.5 | 803.5 | 76.3 | 170.1 | 5.3 | |||
| 30 mg/kg | single dose oral with plant decoction | 139.3 | 3 | 3.6 | 678.2 | 731.7 | 42.9 | 248.1 | 9.7 | |||
| wistar rat (male, 260 ± 20 g) | 6 mg/kg | IV | 8517.80 | 0.1 | 0.66 | 2656 | 2674.70 | 2.3 | – | 3.7 | [69] | |
| 40 mg/kg | single dose oral | 299.2 | 0.33 | 1.94 | 614.8 | 651.7 | 62.4 | – | ||||
| kunming mice (male and female) | 12.02 mg/kg | single dose oral with plant decoction | – | – | – | 1.4 | – | – | – | – | [143] | |
| laurolitsine | rat (male, 200 ± 20 g) | 2 mg/kg | IV | 100.1 | 0.08 | 1.67 | 50 | 52.29 | 38.96 | 86 | 18 | [86] |
| 10 mg/kg | single dose oral | 14.1 | 0.47 | 3.73 | 40.6 | 47.77 | 218.26 | 1207.6 | ||||
| isoboldine | SD rat (male, 230 ± 10 g) | 10 mg/kg | IV | – | 0.3 | 1.2 | 1.2 | 0 | – | 1.4 | [85] | |
| 30 mg/kg | single dose oral | 111 | 0.31 | 0.6 | 0.05 | 0.05 | 19.7 | – | ||||
| roemerine | SD rat (male, 200 ± 20 g) | 6 mg/kg | IV | 1835 | 0 | 1.8 | – | 1631 | 3.8 | 9.4 | 84 | [41] |
| 20 mg/kg | single dose oral | 1358 | 0.2 | 1.6 | – | 4541 | 4.4 | 10.2 | ||||
| isocorydine | SD rat (male and female) | 2 mg/kg | IV | 1843.3 | 0.03 | 1.2 | 831.9 | 857.4 | 2.4 | 4.3 | 34 | [70] |
| 20 mg/kg | single dose oral | 2496.8 | 0.3 | 0.9 | 2848.1 | 2865.3 | 7.2 | 9.3 | ||||
| 6-Odemethylmenisporphine | SD rat (male, 230 ± 10 g) | 0.1 mg/kg | IV | 1556 | 0.1 | 4.5 | 4117 | 4201 | – | – | 52 | [71] |
| 1 mg/kg | single dose oral | 2201 | 2.8 | 5.1 | 21,215 | 21,506 | – | – |
-: not detected; PD: Parkinson’s disease; IV: intravenous injection; Cmax: maximum plasma concentration; Tmax: time to reach Cmax; T1/2: terminal elimination half-life; CL: clearance rate, Ebody = CL/cardiac output, above 0.35, around 0.15, around 0.05 was considered as high, medium and low elimination rate, respectively; Vd: apparent volume of distribution; The oral absolute bioavailability: ; : the total area under the plasma concentration–time curve from 0 h to the last measured concentration,: the area to infinite.
Table 3.
Main metabolic pathways and related enzymes of natural aporphine alkaloids.
| Natural Compound | Structure | Phase I reaction | Phase II reaction | Ref. |
|---|---|---|---|---|
| Apomorphine | 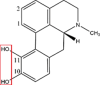 |
Oxidation: OH→=O | Glucuronidation: 10- and 11-O-monoglucuronides O-methylation: OH → OCH3 (COMT) |
[76] |
| Nuciferine | 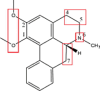 |
N-oxidation: (CYP 3A4); Demethylation: OCH3 → OH (CYP 1A2, 2A6, and 2C8)Dehydrogenation: C4-C5 and C6a-C7 (CYP 3A4) Hydroxylation: C5 → –OH (CYP 3A4) |
N-glucuronidation (UGT1A4) | [65], [144] |
| Magnoflorine | 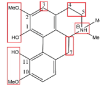 |
O-demethylation: 2,10-OMe N-demethylation Dehydrogenation: C4-C5 and C6a-C7, O-ketonization: 4-OHHydroxylation: C-3, C-4, C-9 |
O-glucosylation, O-glucuronidationO-sulfation |
[68] |
| Dicentrine | 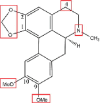 |
N-oxidationN or O or O, O-demethylation (1-OCH2O-2)Hydroxylation: C-3, C-4 |
Glucuronidation Glucosylation O-methylation: OH → OMe (C-4) |
[82] |
| Boldine | 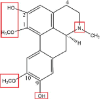 |
N-demethylation (N-demethyl-boldine-O-sulphate) | Glucuronidation and Sulphation(boldine-O-glucuronide, boldine-O-sulphate and disulphate, boldine-O-glucuronide-O-sulphate) | [83] |
| Isoboldine | 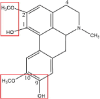 |
– | Glucuronidation Sulfonation: OH → SO3H |
[85] |
| Norisoboldine | 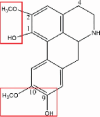 |
– | Glucuronidation: OH → GluA, Sulfonation: OH → SO3H (noriosboldine-1-O-β-d-glucuronide, norisoboldine-9O-α-d-glucuronide and disulfuric acid-1, 9-norisoboldine ester) |
[84] |
| Thalicarpine | 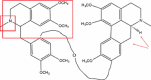 |
N-demethylation Aporphine ring oxidation Benzylic oxidationbenzylic reduction |
– | [87] |
COMT: catechol-O-methyl transferase; CYP: Cytochrome P450 enzymes; UGT: UDP-glucuronosyltransferases.
Absorption
The absorption rate of AAs is highly associated with the maximum plasma concentration (Cmax) and time to reach the concentration peak (Tmax). The clinical absorption of apomorphine and nuciferine into the bloodstream is shown in Table 2. Clinically, the oral administration of apomorphine, a plant-derived AA generally used as an adjunctive medication in Parkinson's disease patients, was limited by its first-pass metabolism with low bioavailability (F%) < 4 % [60]. Therefore, the apomorphine sublingual film, subcutaneous apomorphine injection, and subcutaneous apomorphine prefilled injection pen were developed and showed high absorption rate in plasma, with Cmax > 10 ng/mL and Tmax < 0.8 h [60]. Subsequently, both the subcutaneous apomorphine injection (3, 4, 5 mg/kg with Cmax of 6, 10.3, and 12.4 ng/mL) and subcutaneous apomorphine prefilled injection pen (3, 4, and 5 mg/kg with Cmax of 7, 11.5, and 16.8 ng/mL) resulted in a dose-dependent systemic exposure (Cmax) and high oral absolute bioavailability (F value, 98 % and 83 %, respectively), while a low F value (17 %) was found using the sublingual film administration (Table 2), suggesting that the subcutaneous injection (SC) of apomorphine was more suitable for the clinical treatment [60]. Besides, nuciferine, as the pharmacokinetics marker of Tangzhiqing tablet used in patients, showed a dose-dependent blood absorption (1.28, 1.92, and 2.56 g Tangzhiqing tablet with Cmax of 1.6, 2.1, and 3.8 ng/mL) with Tmax < 2 h, which was attributed to its high permeable property [61].
Nuciferine used in vivo also exhibited rapidly absorption into the blood (Tmax < 1 h) and a dose-dependent systemic exposure. Nuciferine oral administration at the doses of 2, 5, 10, 22.5, and 22.8 mg/kg was rapidly distributed in the plasma, with a maximum concentration (13.5, 63.4, 103, 1710, and 317.6 mg/kg) after 0.9, 0.6, 0.5, 0.9, and 3.5 h, respectively, indicating the close association between absorption rate and administration dose (Table 2) [62], [63], [64], [65]. The oral absolute F value of nuciferine was approximately 4 % with 10 mg/kg and 58 % with 22.5 mg/kg through oral administration [66], indicating that the F of nuciferine had dose-dependent effects. And the F value was increased by 3.3 ± 0.61-fold in nuciferine-loaded poly lactic-co-glycolic acid nanoparticles as compared to nuciferine alone [67]. Another two lotus alkaloids called N-nuciferine and roemerine were also rapidly absorbed into the blood and reached the Cmax (570 ng/mL and 1358 µg/mL, respectively) at 1.65 h and 0.22 h, with a good oral F value (79.9 %, and 84 %, respectively) [41], [66].
The pharmacokinetics of magnoflorine, a naturally occurring simple AA, has been reported in many studies using rat and mouse models. Xue et al. [68] found that the F value of magnoflorine decreased from 8.9 % to 5.3 % as the concentration increased from 15 to 60 mg/kg, but subsequently increased to 9.7 % after the gavage of coptidis rhizoma decoction (equivalent to 30 mg/kg of magnoflorine), suggesting that the remaining substances of decoction performed a synergistic effect with magnoflorine absorption. Tian et al. [69] revealed that the coadministration of magnoflorine and berberine also enhanced the F value of magnoflorine. Besides, both simple aporphine isocorydine and oxoisoaporphine 6-O-demethylmenisporphine underwent rapid (Tmax < 0.3 h and 3 h) and high absorption rate (F = 33.4 % and 51.5 %) after the administration of 20 mg/kg and 1 mg/kg, respectively, which was related to their small molecular weight (Mw 〈4 0 0), high lipophilicity and permeability [70], [71].
Distribution
Clinically, apomorphine quickly crossed the blood–brain barrier (BBB) and both enantiomers of apomorphine equally and uniformly concentrated in different brain tissues, showing neurotropic properties. Moreover, apomorphine quickly crossed the nasal and intestinal mucosa, thus nasal and rectal administration were used for disease treatment [60].
The distribution of AAs was highly associated with apparent volume of distribution (Vd). A wide tissue distribution of nuciferine has been found in vivo, with a Vd > 50 L/kg, mainly accumulated in the kidney and lung, followed by spleen, liver, brain, heart, and adipose tissue after oral administration, and similar distribution of N-nuciferine and roemerine has been found [41], [62], [63], [65], [66]. The high concentration in the lung, kidney, and spleen was possibly attributed to the high blood flow in these organs, and the presence of nuciferine in the liver and adipose tissue provided a proof for its anti-obesity and hypolipidemic effects [62]. The low F-value and high accumulation of nuciferine in the kidney may be an obstacle to developing an oral formulation for long-term use, as it presents a potential risk of kidney toxicity.
Magnoflorine, generally isolated from the herbal medicine Coptis chinensis Franch., was widely distributed in the liver, followed by spleen and lung, and the lowest in the brain [72]. The significant difference of Vd values observed in the oral administration of the coptidis rhizoma decoction (Vd = 248.1 L/kg) and magnoflorine alone (Vd = 102.6–170.1 L/kg) highlighted the importance of considering the co-treatment of magnoflorine with other phytochemicals for human health [68].
Taspine was generally used to enhance wound healing and both taspine solution and taspine liposome demonstrated a wide distribution in the liver, spleen, lung, kidney, heart, and penetrate through the BBB. The administration through liposome obviously prolonged the residence time in the systemic circulation, increased the liver concentration, and decreased the heart accumulation [73]. 6-O-demethylmenisporphine, an oxoisoAA extracted from Menispermi Rhizoma, was also widely distributed in the kidney, heart, lung, liver, muscles, spleen, and brain. According to experimental data from urine, bile, and feces, the prototype drug (6-Odemethylmenisporphine) was completely excreted at 24, 24, and 12 h, respectively, suggesting that it was widely metabolized in vivo and the metabolites were subsequently eliminated [71].
The permeability of compounds from the blood system to the tissues for their distribution is determined by their physicochemical properties, especially liposolubility. The brain distribution of AAs also plays an important role in the regulation of the central nervous system. Apomorphine, nuciferine, N-nuciferine, and roemerine rapidly crossed the BBB due to the simple diffusion related to the high lipophilicity at physiological pH, contributing to exert sedative-hypnotic and anxiolytic effects in the brain [66]. Besides, effective apomorphine-loaded nanoparticles have been designed for improving the brain accumulation of apomorphine [74]. The similar structure between isocorydine and apomorphine indicated that isocorydine effectively crossed the BBB, implying its central pharmacological activity [70]. 6-Odemethylmenisporphine exerted a potential therapeutic effect on nervous disorders by enhancing the cholinergic neurotransmission and reducing acetylcholinesterase aggregation [71]. Due to its weak neuroregulatory effect, alternative strategies are required to enhance the liposolubility and permeability of magnoflorine, ultimately improving its neuroprotective activity [75].
Metabolism
AAs are catalyzed by various enzymes in the body, especially cytochrome P450 (CYP) and uridine diphosphate glycosyltransferase (UGT), and participate in multiple chemical reactions in the metabolic process, which commonly include two steps (Fig. 3). On the first step, oxidation, reduction, and hydrolysis reactions introduce polar groups (for example –OH, -SH, and –NH2) to the functional groups for enhancing the water solubility of metabolites. On the second step, the obtained metabolites are bound to endogenous substances to produce more hydrophilic compounds, further excreted through the kidney, intestine, and other ways. Table 3 shows the metabolic pathways of AAs.
Apomorphine was easily and mainly (auto)oxidized, followed by glucuronidation and sulfation in patients with Parkinson's disease [76]. The phase II metabolites were 10- and 11-O-monoglucuronides as well as apocodeine, and a small amount of isoapocodeine was generated through the catechol-O-methyl transferase, as shown in Table 3. Traditional Chinese Medicine K-601 has been studied in pharmacokinetics research involving six healthy males. Magnoflorine, one of the major compounds in K-601, underwent primarily phase I metabolism (specifically demethylation) and phase II metabolism (including glucuronidation, acetylation and sulphation) [77]. Similar results were found in both in vivo and in vitro studies, showing that 12 metabolites of magnoflorine have been detected (8 phase I metabolites originated from N or O-demethylation, O-ketonization, dehydrogenation and hydroxylation and 4 phase II metabolites originated from O-glucosylation, O-glucuronidation and O-sulfation) (shown in Table 3) [68]. Gut microbiota and their metabolites enzymatically transform natural products to affect compounds pharmacokinetics and bioavailability profiles. Phase I biotransformation of magnoflorine is activated by anaerobically incubation magnoflorine with gut microbiota (the supernatant of rats’ fresh feces) in vitro [68].
A comprehensive metabolic generation mechanism of nuciferine was elaborated in vivo. 55 metabolites were identified from mice, involving 29 phase I metabolites and 26 phase II metabolites [78]. To further examine the metabolic mechanism of nuciferine, the enzymes’ catalytic activities were evaluated in vitro incubation systems. As shown in Table 3, the main phase I enzymes including CYP1A2, CYP2A6, CYP2C8 for demethylation, and CYP3A4 for N-oxidation, dehydrogenation and hydroxylation, and the phase II enzymes including UGT1A4 and UGT1A9 for N-glucuronidation, as well as the sulfated-related enzymes SULT1A1″1 and SULT1A1′′2 confirmed high catalytic activities for metabolites formation of nuciferine [78], [79]. The molecular docking analysis was also identified the interaction between nuciferine and CYP isoforms [78]. Although nuciferine is catalyzed by many enzymes, it competitively inhibited CYP2D6 activity with N-nornuciferine and 2-hydroxy-1-methoxyaporphine due to their aporphine parent nucleus, resulting in potential adverse drug interactions in the clinic, which should be further studied [80]. Meanwhile, the gut microbiota Akkermansia, Lactobacillus, and Bifidobacterium were possibly involved in the metabolism of nuciferine in gestational diabetes mellitus mice, but the understanding of how gut microbiota directly and indirectly affect AAs metabolism is limited studied [81].
Table 3 showed that the metabolic profile of dicentrine, a simple AA rich in a variety of genera in the plant family Lauraceae, consists of 24 metabolites characterized in pig urine, including 9 phase I and 15 phase II conjugates, and the phenolic group of dicentrine underwent glucuronidation and glucosylation while no sulfation was observed after phase I biotransformation [82]. As one of the simple aporphines and most potent natural free radical scavengers, boldine was identified as the main compound bio-transformed through N-demethylation in phase I and subsequently through O-conjugation with glucuronic and sulphuric acid in phase II [83]. The free hydroxyl groups in the boldine were directly available for conjugation and they were beneficial for performing phase II bio-transformation.
Boldine, isoboldine, and norisoboldine have a similar structure, implying the similar pharmacokinetic and metabolic profiles in rat plasma, bile, urine and feces, such as the common glucuronidation and sulphation in phase II bio-transformation [84], [85]. Therefore, a reasonable hypothesis was proposed that isoboldine and norisoboldine may also undergo N-demethylation during their phase I metabolism. As shown in Table 3, the major glucuronidation and sulphation generally occurred on –OH group of three AAs. The mentioned metabolic pathways may also be suitable for laurolitsine due to a similar structure of laurolitsine with isoboldine [86]. Thalicarpine, a dimeric AA consisting of aporphine and benzyltetrahydroisoquinoline units, was metabolized through three pathways, such as N-demethylation, aporphine ring oxidation, and benzylic oxidation/reduction (phase I). Only one metabolite was identified as glaziovine glucuronide found in urine samples [87].
In summary, it is likely that most AAs shared common characteristics such as the high absorption (generally Cmax > 10 ng/L, Tmax < 3 h) and elimination (generally T1/2 < 3 h, CL > 50 L/kg/h) rate, and the nonspecific biodistribution, which can be attributed to their small molecular weight, nonpolar hydrocarbon backbones and hydrophobic functional groups like aromatic rings. The liver and the gut play an important role in drug metabolism, including methylation, N- or O-demethylation, O, O-demethylenation, N-oxidation and reduction for phase I metabolic transformation, and N- or O-glucuronidation, O-glucosylation, sulphation, and sulfonation for phase II metabolic transformation, which was regulated by CYP and UGT enzymes. Similar metabolites were found in both in vivo bio-samples and in vitro incubation systems of AAs and gut microbiota during AAs metabolism, suggesting a potential influence of gut microbiota on this process. However, as the metabolic effects of the gut microbiota are rarely studied in AAs pharmacokinetic research, the contribution of the gut microbiota to AAs metabolism is probably underestimated. Additionally, while most studies are now characterizing the metabolites derived from biotransformation of AAs, these data remain pre-clinical in nature, and more clinically actionable insights in specific pharmacokinetic parameters of AAs should be reported in future.
Safety
The toxicities of AAs are tightly linked with the dosage and administration methods, such as oral, SC, and intraperitoneal injection (IP). A mixture of Chinese herbal medicines containing nuciferine (0.9 mg/g), nornuciferine (0.3–0.4 mg/g), and O-nornuciferine (0.3 mg/g) was administered under the oral treatment of 4.38 g/day Hedan Tablets to patients with hyperlipidemia for 8 weeks. The levels of the proprotein convertase subtilisin/kexin type 9 (a circulating protein that is negatively correlated with drug efficacy) were not altered, and no change was also found in the levels of alanine aminotransferase and aspartate aminotransferase, indicating the Hedan Tablets exert beneficial effects without evident toxicities [88]. The subacute toxicity test confirmed the safety of magnoflorine in a certain dose range in mice. No evident toxicity was observed after 4 weeks of continuous intragastric administration at a dose of 100 mg/kg and 200 mg/kg. No significant reaction was observed after the administration of 40 mg/kg magnoflorine by gavage in cats for 4 h.
The most common administration route for apomorphine is SC due to its low bioavailability (<4%) in oral therapy. The primary administration route in Parkinson's disease includes continuous subcutaneous apomorphine infusion and intermittent injections. The usual dose of apomorphine in patients was 2–6 mg/kg, with a total of 88 mg (range 24–340 mg) as daily clinical application [89]. The adverse event rate of continuous subcutaneous apomorphine infusion, which reduced the duration (-50 %) and the severity (-45 %) of the dyskinesias, was usually lower than that after intermittent injections, promoting the duration (+33 %) and the severity (+14 %) of the dyskinesias [90]. The most widespread side effects were subcutaneous nodules, nausea, and somnolence. Apomorphine therapy caused infrequent immune hemolytic hematologic anemia at the dose of 75 mg/d [91]. Other ways of apomorphine administration include buccal, inhalation, and pump-patch routes, which were promising delivery strategies as a substitution therapy for patients with needle phobia [60].
An IP of 50 mg/kg magnoflorine in mice significantly increased the plasma levels of IL-6 and IL-1β, and exerted a negative effect on neuronal Ca2+ metabolism, subsequently leading to neurotransmission disorders. On the contrary, no significant changes were observed in the levels of interleukin (IL) 1β, IL-6 or tumor necrosis factor α (TNF-α) after a low dosage through IP of magnoflorine (10 and 20 mg/kg) [92]. The median lethal dose of N-nornuciferine with lower levels of hyperlipemia and cholesterol, was 323 μg/kg (IP) in mice [93]. Boldine, administered at a dose of 100 mg/kg through IP to rats for 14 days, resulted in no mortality or any onset of clinical or toxicological symptoms throughout the study period [94].
The toxicity of AAs has been studied on normal cell lines in vitro as well. The cytotoxicity of oxoglaucine, an oxoaporphines originated from herbal medicine, against two human normal cell lines HL-7702 and HUVEC was 40.2 ± 1.0 μM (IC50) and 90.2 ± 1.3 μM (IC50), respectively, indicating its low toxicity [95]. Besides, two dehydroAAs isolated from the roots of Thalictrum foetidum, such as 9-(2′-formyl-5′,6′-dimethoxyphenoxy)-1,2,3,10-tetramethoxy dehydroaporphine and 9-(2′-formyl-5′, 6′-dimethoxyphenoxy)-1, 2, 3, 10-tetramethoxy oxoaporphine, exhibited an antitumor effect against glioblastoma stem cells 3 times higher than that on the human normal cell line (293 T, IC50 = 15.5 and 8.2 µg/mL, respectively), suggesting its significant selective cytotoxic effect on different cells [93]. Toxicity studies suggested that most AAs had non-toxic or low-toxicity properties. However, long-term and high-dose safety tests in clinical practice are still lacking.
Anticancer activities of AAs on cancer
Anti-cancer effects of AAs on multiple cancer types, including hepatocellular carcinoma, gastric, colorectal, pancreatic, and lung cancer, have been widely studied (Table 4). Although few clinic trials are reported so far, abundant evidence shows significant anti-cancer effect after a treatment with AAs alone or a synergistic treatment with metal/clinical therapeutic drugs.
Table 4.
Anticancer activities of aporphine alkaloids on various cancer types.
| Cancer type | Constituents | Model/Cell (IC50) |
Dose | Effects | Mechanisms | Ref | |
|---|---|---|---|---|---|---|---|
| In vivo | Hepatocellular carcinoma | Isocorydine | Murine Model with SP cells from MHCC-97L cells | 0.4 mg/kg (IP) | ↓Tumor volume; ↓Tumor weight | ↑Fas; ↑Bax; ↑Bim; ↑Bik; ↑Bak; ↑puma;↓Bcl-2; ↓Bcl-xl; ↓Mcl-1 | [99] |
| Isocorydine | Murine Model with Huh7 or SMMC-7721 cells | 0.4 mg/kg (IP) | ↓Tumor volume; ↓Tumor weight | ↑p-Chk1; ↑p-Chk2 | [119] | ||
| 8-acetamino-isocorydine | Murine Model with H22 cells | 100 mg/kg (oral) | ↓Tumor umor volume; ↓Tumor weight | – | [35] | ||
| 8-amino- isocorydine | Murine model with HCC-LY5 cells | 0.12 mg/mice (IP) | ↓Tumor weights; ↓Tumor volumes | ↑C/EBPβ | [100] | ||
| Boldine | Rats model treated by diethylnitrosamine | 90 mg/kg (oral) | ↑Liver weight | ↓Bcl2; ↑Bax; ↑cytochrome c; ↑cleaved caspase 3 | [94] | ||
| Oxoglaucine | Murine model of hepatoma with BEL-7402 cells | 75 mg/kg (IP) | ↑Tumor growth inhibition rate | – | [95] | ||
| Chiral platinum (II)-4-(2,3-dihydroxypropyl)-formamide oxoaporphine | Murine model with BEL-7404 and BEL-7402 cells | 8 mg/kg (IP) | ↓Tumor growth | ↓hTERT; ↓ c-myc | [103] | ||
| cobalt oxoisoaporphine complexes | Murine model with BEL-7402 and T-24 cells | 5 mg/kg (IP) | ↓Tumor growth | ↑ROS; ↑Apaf-1; ↑cytochrome c; ↑caspase-3/9 | [145] | ||
| Oxoaporphine-Zn complexes | Murine model with HepG2 cells | 6 mg/kg (IP) | ↓Tumor weights; ↓Tumor volumes | ↑p53; ↑p21; ↑p27; ↑Chk1; ↑Chk2 | [105] | ||
| Magnoflorine | Murine model with SGC7901 cells | 10 mg/kg (IP) | ↓Tumor cell density | ↓AKT; ↑JNK | [110] | ||
| Colorectal cancer | Nuciferine | Murine model with CT26 cells and SY5Y cells | 1 mg/mL (IP) | ↓Tumor weights | ↓PI3K-AKT and IL-1β levels | [96] | |
| Organoplatinum (II) complexes with Oxoisoaporphine | Murine model with NCI-H460 cells | 6 mg/kg (IP) | ↓Tumor weights; ↓ volumes | ↑Caspase-3/9; ↓Telomerase | [104] | ||
| Pancreatic cancer | Isocorydine | murine model with PANC-1 cells | 0.4 mg/kg (IP) | ↓Tumor weights; ↓Tumor volumes | ↓p-STAT3 | [106] | |
| Nuciferine | Murine model with PANC-1 cells | 30 mg/kg (IP) | ↓Tumor volume; ↓Tumor weight | ↓pYAP | [107] | ||
| Lung cancer | Nuciferine | Murine model with A549 cells | 50 mg/kg (IP) | ↓Tumor weight; ↓Tumor size | ↑Axin; ↑Bax; ↓β-catenin; ↓Bcl-2 | [97] | |
| Nuciferine | Murine model with LA795 cells | 30 μM (oral) | ↓Tumor growth | ↓TMEM16A | [2] | ||
| Nuciferine | Murine model with A549/T cells | 7.5 mg/kg (IP) | ↓Tumor growth; ↓Tumor size | ↓PI3K/AKT | [108] | ||
| Glioblastoma | Nuciferine | Murine model with U251 cells | 15 mg/kg (IP) | ↓Tumor size, ↓angiogenesis; ↓hardness index | ↓p-AKT; ↓p-STAT3; ↓SOX2 | [120] | |
| Breast cancer | Boldine | Rat model with LA7 cells. | 100 mg/kg (IP) | ↓Tumor growth | ↑caspase-3/7; ↓NF-κB | [111], [114] | |
| KO-202125 | Murine model with MDA-MB-231 cells | 1.95 mg⁄kg (oral) | ↓Tumor growth | ↓AKT | [111] | ||
| Magnoflorine | Murine model with MCF-7 cells. | 3 mg/kg (IP) | ↓Tumor volume; ↓Tumor weight | ↑caspase-3; ↑LC3-II; ↑p-p38 | [20] | ||
| 7-hydroxydehydronuciferine | Murine model with A375.S2 cells | 20 mg/kg (IP) | ↓Tumor size | ↑PI3k/AKT | [121] | ||
| Apomorphine | Zebrafish transgenic model | 50 μM | ↓Vascular formation | – | [19] | ||
| In vitro | Hepatocellular carcinoma | Magnoflorine | HepG-2 (0.4 μg/mL)U251 (7 μg/mL) |
1–10 μg/mL | ↓Cell viability | – | [39] |
| Lanuginosine | HepG-2 (2.5 μg/mL)U251 (4 μg/mL) |
1–10 μg/mL | |||||
| Xylopine | HepG-2 (6.31 μM) | 5, 10 μg/mL | ↑Cytotoxicity | ↑G2/M arrest | [101] | ||
| Xylopine | HepG-2 (9.4 μM) | 14 μM | ↑Cytotoxicity | ↑G2/M phase arrest; ↑p53-independent pathway | [122] | ||
| Boldine | HepG-2 (55.66 ± 1.3 μM) | 50 μg/mL | ↓Cell viability | ↑G2/M arrest, ↑caspase-9 and caspase-3/7 | [125] | ||
| 8-hydroxyartabonatine C | HepG-2 (12.9 μM) | 60 μM | ↑Cytotoxicity | – | [135] | ||
| ouregidione | HepG-2 (26.3 μM) | 60 μM | ↑Cytotoxicity | – | |||
| Platinum (II) complex of Liriodenine | BEL-7404 (6.9 μM) | 10 μM | ↓Cell proliferation | ↑S/G2/M phase arrest; ↓Telomerase | [103] | ||
| Gastric cancer | 7-hydroxydehydronuciferine | AGS (62.9 μM) | 100 μM | ↓Cell proliferation | – | [41] | |
| Colorectal cancer | Xylopine | HCT-116 (6.4 μM) | 14 μM | ↑Cytotoxicity | ↑G2/M phase arrest; ↑p53-independent pathway | [122] | |
| D-dicentrine analogues | COLO 201 (3.9 μM) | 10 μM | ↑Apoptosis | ↓TOP2; ↑G2/M arrest | [146] | ||
| Lung cancer | Crebanine | A549 (25 μg/mL) | 5–15 μg/mL | ↓Proliferation; ↓Migration | ↑TNF-α; ↓NF-κB;↓Bcl-2 | [147] | |
| Thailandine | A549 (0.30 μg/mL) | 50 μg/mL | ↑Cytotoxicity | – | [139] | ||
| Magnoflorine with cisplatin | NCIH-1277 (36.5 µg/mL) | – | ↓Proliferation; ↓Migration | ↑p53; ↑caspase-3; ↑LC3-II; ↑p-p38;↓p-AKT | [118] | ||
| Laryngeal Squamous Cell Carcinoma | Xylopine | SCC9 (26.6 μM); HSC3 (15.7 μM) | 14 μM | ↑Cytotoxicity | ↑G2/M phase arrest; ↑ p53-independent pathway | [122] | |
| Glioblastoma | Magnoflorine | U251(7 μg/mL) | 1.1 mg/mL | ↓Cell viability | – | [39] | |
| lanuginosine | U251 (4 μg/mL) | 1.1 mg/mL | ↓Cell viability | – | |||
| AAs isolated from Thalictrum foetidum | GSC-3#, GSC-18# (2.36–5.37 μg/mL) | 0.5–60 μg/mL | ↓Cell viability | – | [93] | ||
| Breast cancer | Boldine | MCF-7 (160 µM) | 63–2000 μM | ↓Proliferation | ↓Telomerase | [125] | |
| N-benzylsecoboldine | MCF7(16.25 µM); MDA-MB-231 (21.88 µM) | – | ↓Cell viability | ↓Telomerase | [124] | ||
| Crebanine | MCF-7 cells (27 μg/mL)MDA-MB231 (15 μg/mL) |
5–15 μg/mL | ↓Cell viability | ↑TNF-α; ↓NF-κB;↓Bcl-2 | [147] | ||
| Oxocrebaine | MCF-7 (16.66 μM) | 4–64 μmol/L | ↓Proliferation; ↓migration | ↑DNA damage; ↑Autophagy | [126] | ||
| Four AAs isolated from Stephania venosa Spreng | MDA-MB231 (4–40 μg/mL) | 1 mg/mL | ↓Proliferation | ↑G2/M arrest; ↑Apoptosis | [139] | ||
| Two AAs isolated from Fissistigma glaucescens | MCF-7 (3.2–8.1 μg/mL) | – | ↑Cytotoxicity | – | [43] | ||
| Xylopine | MCF-7 (12 μM) | 14 μM | ↑Cytotoxicity | ↑G2/M phase arrest; ↑ p53-independent pathway | [122] | ||
| Four AAs isolated from Stephania venosa Spreng | MCF-7 (22.2 μM; 23.9 μM) | 1 mg/mL | ↑Cytotoxicity | – | [139] | ||
| Melanoma | N-methylated nuciferine N-methylasimilobine(-) -lirinidine2-hydroxy-1-methoxy-6a,7-dehydroaporphine |
Melanogenesis 4A5 cells (15.8 μM; 14.5 μM; 19.3 μM; 13.3 μM) | 3–30 μM | ↓Melanogenesis | ↓Tyrosinase; ↓TRP-1; ↓TRP-2 | [42] | |
| Epithelial ovarian cancer | Liriodenine | CAOV-3 (37.3 μM) | 20–40 μM | ↓Cell viability | ↑S phase arrest; ↑caspase-3; ↑caspase-9 | [25] | |
| Osteoma cells | Magnoflorine with cisplatin | MG-63 (2.05 μM); U-2 osteoma (4.81 μM) | – | ↓Proliferation; ↓Invasion | ↑HMGB1; ↑ NF-κB; ↑ miR-410-3p | [118] | |
| Leukemia | Xylopine | HL-60 (18.5 μM)K562 (7.8 μM) |
14 μM 14 μM |
↑Cytotoxicity | ↑G2/M phase arrest; ↑ p53-independent pathway | [122] |
AKT: protein kinase B; AMPK: AMP-activated protein kinase; Apaf-1: the apoptotic protease activating factor-1;
CDK1/2: cyclin dependent kinases 1/2; hTERT: human telomerase reverse transcriptase; IP: intraperitoneal injection; IL: interleukin; JNK: stress-activated protein kinases; NF-κB: nuclear factor kB; PI3K: phosphatidylinositol 3-kinase; ROS: reactive oxygen species; STAT3: activator of transcription 3; SOX2: sex determining region Y (SRY)-box 2; TNF-α: tumor necrosis factor α; TMEM16A: the transmembrane protein 16A; YAP: YES-associated protein Ser127.
Anti-cancer effect of AA alone
Nuciferine, the main simple AA present in lotus leaf, was used in a murine subcutaneous transplantation model with colorectal carcinoma CT26 cells and SY5Y cells at a dose of 9.5 mg/kg, IP, and the results show that the tumor weight is significantly smaller than that of the control group. Notably, the anticancer activity in the group of mice treated with nuciferine soon after tumor implantation is higher than that in the mice treated with nuciferine when the tumor reached a size of 100 mm3. [96] Nuciferine (50 mg/kg, IP) in the presence of nicotine significantly inhibits tumor weight and size in the murine subcutaneous transplantation model with lung adenocarcinoma A549 cells, virtually eliminating the proliferative effect of nicotine in non-small cell lung cancer [97]. Nuciferine significantly inhibits laryngeal squamous cell carcinoma survival in a concentration-dependent manner by inhibiting the Tripartite Motif Containing 44 mRNA levels and inhibiting the activation of Toll-like receptor 4 (TLR4) downstream kinases such as protein kinase B (AKT) signaling pathway, which is detected in the murine subcutaneous transplantation model with laryngeal squamous cell carcinoma TU212 cells [98]. The effect of 1 mg/mL nuciferine treatment at an early stage of carcinogenesis is superior to that at a middle stage in the murine subcutaneous transplantation model with neuroblastoma SY5Y cells [96]. In the transplantation model with glioblastoma U251 cells, the tumor weight and size of the 15 mg/kg nuciferine-treated group is significantly smaller than those in the control group, and tumor progression markers Ki67, CDC2, Bcl-2, HIF1α, and N-cadherin are decreased in the nuciferine-treated group [75].
The antitumor effect of isocorydine, a simple aporphine with a 5, 6, 6a, 7-tetrahydro-4H-dibenzo quinoline core, was studied in a liver cancer model induced using hepatocellular carcinoma side population cells. The treatment with isocorydine (0.4 mg/kg) significantly reduces tumor weight by 50 % compared to that of the vehicle group in side population cell-induced mice, whereas no significant difference in tumor size and tumor weight is found between isocorydine and vehicle treatment in the non-side population group, indicating that isocorydine selectively inhibits the growth of side population cells [99]. The results are confirmed by a murine subcutaneous transplantation model of hepatoma using hepatocellular carcinoma Huh7 or SMMC-7721 cells [100].
Boldine, isolated from the bark and leaves of the Boldo tree (Peumus boldus), also shows excellent antiproliferative and apoptotic properties on cancer cells. Oral supplementation with boldine (90 mg/kg) in diethylnitrosamine induced rats of liver cancer significantly decreases liver weight as well as the serum tumor markers alpha‐fetoprotein and carcinogen embryonic antigen [94]. The oxoaporphine 7-hydroxydehydronuciferine inhibits malignant melanoma in mice subcutaneously transplanted with melanoma A375.S2 cells.
AAs inhibit cancer cell growth in vitro. Magnoflorine and lanuginosine, which were separated from the methanol extract of magnolia leaves, exert a significant cytotoxicity on HEPG2 cells, with an IC50 value of 0.4 and 2.5 μg/mL, respectively [39]. Nineteen alkaloids were isolated from the stem of Xylopia laevigata, and most of these alkaloids exert a significant cytotoxicity against HepG2 cells, with IC50 values below 15 μg/mL, indicating the high inhibition rate. Remarkably, (+)-xylopine shows the strongest cytotoxic activity, with a IC50 value of 1.87 μg/mL in HepG2 cells [101]. Most of the studies are based on the separation and purification of aporphines followed by cancer inhibition experiments without further confirmation in vivo studies. The specific AAs tested in vivo and in vitro are listed in Table 4.
Structure-anticancer activity relationship: Role of the C8 position of AAs
Previous studies showed that AAs possess a relatively planar conformation, readily inserted into the DNA double helix, promoting DNA sequence changes, stimulating DNA strand breaks, or inhibiting DNA topoisomerase II activity [102]. The target modified product is 8-amino-isocorydine, which is obtained by the replacement of an amide group at C8 on simple AAs to maintain the more stable planar conjugate configuration, further modified as 8-acetamino-isocorydine due to its instability in aqueous solution. The prodrug 8-amino-isocorydine (100 mg/kg) has a significant antitumor activity by reducing tumor weight by 53.12 % in the murine transplantation model with hepatocellular carcinoma H22 cells. Although the inhibitory effect of 100 mg/kg 8-amino-isocorydine is not stronger than cyclophosphamide used as the positive group, the average body weight of the cyclophosphamide group is significantly lower than that of the isocorydine group and control group, suggesting its dangerous potential effect in clinical practice.
Besides, a nitric acid group and a weak electron-donating group were introduced at the C8 position. The compound with a strong electron-donating group (–NH2) at the C8 position has better anticancer activity than the compound with an electron-withdrawing group (NO2) and a weak electron-donating group at the C8 position [35]. The 8-amino-isocorydine is obtained after reduction of the amide group at C8, which shows inhibitory effect on tumor size in hepatocellular carcinoma mice. The treatment with 0.12 mg/kg 8-amino-isocorydine has similar effects compared to those of 0.2 mg/mouse sorafenib treatment, which is used in clinical practice in liver cancer patients, in the murine subcutaneous transplantation model with hepatocellular carcinoma HCC-LY5 cells [100]. A Treatment with 250 mg/kg/8-amino-isocorydine significantly reduces the size and weight of tumors better than that of 40 mg/kg 5-FU in murine subcutaneous transplantation model with gastric cancer MGC803 cells. Moreover, the body weight is not different from that of the control group, indicating the low toxic effect of 8-amino-isocorydine. These results indicate the important role of the C8 position of aporphines, which may be a target position for drug design and synthesis.
Synergistic anticancer bioactivities
Synergy with metals. Since the successful application of platinum-based anticancer drugs, anticancer metal complexes have attracted great interest. Oxoaporphine and oxoisoAAs isolated from traditional Chinese medicines possess antitumor activity, so these two kinds of aporphines are used as ligands. Over the last decades, platinum (II)-based drugs have been widely used in anticancer chemotherapies. However, they inevitably induce drug resistance, serious side effects, and they are highly toxic. The tumor inhibition rate of the 8 mg/kg chiral platinum (II)-4-(2,3-dihydroxypropyl)-formamide oxo-aporphine complexes used in the murine subcutaneous transplantation model with hepatocellular carcinoma BEL-7404 cells and BEL-7404 cells, reached 46.8 % and 47.1 %, respectively [103]. The organoplatinum (II) complexes with Oxoisoaporphine (Pt2) (6 mg/kg) treatment results in a dose-dependent inhibition of tumor growth with an inhibition rate of 38.5 % and less toxicity than cisplatin in a lung cancer mouse transplantation model [104].
Cobalt, one of the main trace elements in cells, shows anticancer effects. The therapeutic effect of 5 mg/kg cobalt oxoisoaporphine complexes in the murine subcutaneous transplantation model with hepatocellular carcinoma BEL-7402 and T-24 cells is similar to that of cisplatin treatment, with an inhibition ratio of 65.7 %. Zn (II) complexes are synthesized by oxoaporphine derivative (0.03 mmol, 0.0109 g), methanol (1.5 mL), and chloroform (0.5 mL). A total of 6.0 mg/kg oxoaporphine-Zn to the murine subcutaneous transplantation model with hepatocellular carcinoma HepG2 cells is able to control tumor growth with a volume reduction of 55.57 %, and a tumor weight inhibition rate of 32.8 % [105]. It is worth noting that the above-mentioned complexes have a much lower body weight inhibition rate than the drug cisplatin used as a positive sample, indicating a lower toxicity in vivo.
Synergy with clinical therapeutic drugs. AAs have synergistic effects with drugs, reducing drug resistance or increasing sensitization. Gemcitabine is widely considered as a preferential treatment option for locally advanced and metastatic pancreatic cancer. Isocorydine reduces the expression of p-signal transducer and activator of transcription 3 (STAT3) in pancreatic cancer cells induced by gemcitabine, down-regulates the expression of transcription factors and proteins related to epithelial-to-mesenchymal transition (EMT), and inhibits the migration and invasion of tumor cells. The combination of isocorydine (0.4 mg/kg) and gemcitabine (100 mg/kg) significantly inhibits tumor growth in the murine subcutaneous transplantation model with pancreatic cancer PANC-1 cells, with a significantly greater effect than isocorydine or gemcitabine alone [106]. Nuciferine has also anticancer activity when used in synergy with gemcitabine, and it significantly reduces the resistance to gemcitabine. Nuciferine (30 mg/kg) in the murine transplantation model with pancreatic cancer PANC-1 cells reduces the resistance to 20 mg/kg gemcitabine by activating the AMP-activated protein kinase (AMPK)-mediated down-regulation of 3-hydroxy-3-methyl-glutaryl-coA reductase (HMGCR) in pancreatic cancer cells associated with an increase in YES-associated protein Ser127 (YAP) phosphorylation [107].
Although cisplatin is frequently used in the clinical treatment of lung cancer, high dosage is associated with a strong toxicity. The tumor size of a murine subcutaneous transplantation model with lung adenocarcinoma LA795 cells treated with 30 μM nuciferine is significantly reduced, with an inhibition rate of 40.1 %. Notably, the cotreatment of nuciferine (30 μM) and cisplatin significantly increases the tumor inhibition rate (15 mg/kg, 80.4 %), which is higher than that of the treatment of cisplatin alone (15 mg/kg, 72.9 %) [2]. Besides, the resistance to the drug paclitaxel, doxorubicin, docetaxel and daunorubicin in A549/T and HCT-8/T cell lines is reduced by the combination therapy with nuciferine. The inhibition of the phosphatidylinositol 3-kinase (PI3K)/AKT/ extracellular signal-regulated kinases (ERK) pathway, the activation of the nuclear factor E2-related factor 2 (Nrf2) and hypoxia-inducible factor-1α (HIF-1α), and the decrease of the expression of P-glycoprotein (P-gp) and breast cancer resistant protein (BCRP) may contribute to the sensitization of nuciferine to multidrug resistance in various cancers [108]. A stronger tumor suppression effect is associated to the cotreatment of doxorubicin (3 mg/kg) and magnoflorine (3 mg/kg) compared with the effect of doxorubicin (3 mg/kg) alone, achieved by the regulation of the AKT/ mechanistic target of rapamycin (mTOR) and p38 signaling pathways. Notably, magnoflorine alone has no significant anticancer activity and no evident toxicity in vivo, suggesting that magnoflorine has the potential to be used as a sensitizer of doxorubicin in clinical practice [20].
Strategies of AA-delivery system for application
Designation of AAs-loaded nanoparticles as carriers to improve the pharmacological activities of AAs in food and medicine application has paid attention over the last two decades. For example, the nuciferine-loaded poly lactic-co-glycolic acid nanoparticles were constructed for sustainable targeted release of nuciferine. Compared with free nuciferine, the nuciferine-loaded nanoparticles significantly decreased the levels of serum total cholesterol, triglycerides and low-density lipoprotein cholesterol and increased high-density lipoprotein cholesterol, which exhibited improvement of potential bioactivities against hepatocarcinogenesis on high-fat diet-induced hyperlipidemia rat [67]. The magnoflorine-loaded chitosan collagen nanocapsules significantly decrease the level of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, more than free magnoflorine administration in cognitive deficit rats, indicating a great role of nanocapsules on magnoflorine delivery and enhancement of bioactivities against inflammation-driven cancer progression [109].
Mechanism of action of AAs against cancer
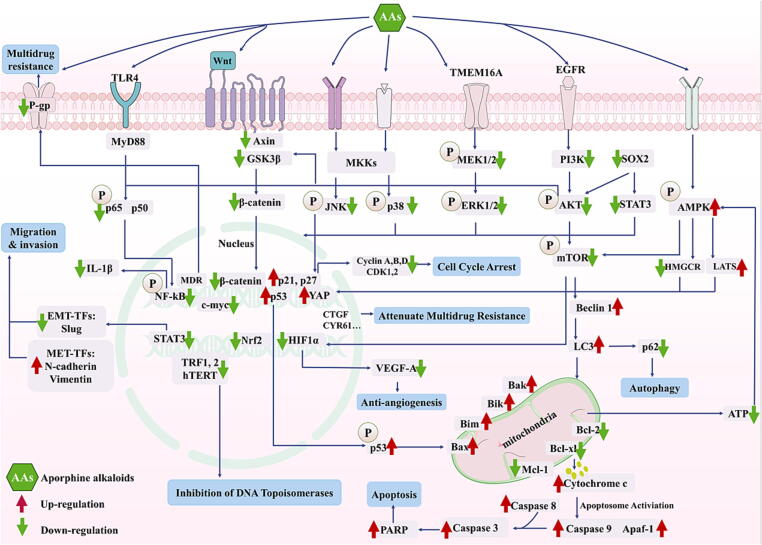
The mechanism of the anticancer action of AAs mainly involves target pathways, including PI3K/AKT, Mitogen-activated protein kinases (MAPKs), Wnt/β-catenin, and nuclear factor kB (NF-κB) and they induce cell autophagy, apoptosis, and cell cycle arrest (Fig. 4).
Fig. 4.
Anticancer mechanisms of AAs. AAs: Aporphine alkaloids; AKT: protein kinase B; AMPK: AMP-activated protein kinase; Apaf-1: the apoptotic protease activating factor-1; CDK1/2: cyclin dependent kinases 1/2; EMT: epithelial-to-mesenchymal transition; ERK: extracellular signal-regulated kinases; EGFR: epidermal growth factor receptor; GSK-3β: glycogen synthase kinase 3β; HMGCR: 3-hydroxy-3-methyl-glutaryl-coA reductase; HIF-1α: hypoxia-inducible factor-1 α; hTERT: human telomerase reverse transcriptase; IL: interleukin; JNK: stress-activated protein kinases; mTOR: mechanistic target of rapamycin; MET: mesenchymal-to-epithelial transition; NF-κB: nuclear factor kB; Nrf2: the nuclear factor E2-related factor 2; PI3K: phosphatidylinositol 3-kinase; P-gp: P-glycoprotein; STAT3: activator of transcription 3; SOX2: sex determining region Y (SRY)-box 2; TLR4: Toll-like receptor 4; TMEM16A: the transmembrane protein 16A; VEGF-A: vascular endothelial growth factor A; YAP: YES-associated protein Ser127.
PI3K-AKT signaling pathway
The activation of the PI3K-AKT signaling pathway is considered as a key factor for cell survival, growth, and resistance to chemotherapy, resulting in cancer development. Particularly, the activated PI3K catalyzes the conversion of PIP2 to PIP3, which results in the activation of AKT by the phosphorylated PIP3, further promoting various biological effects such as cell proliferation and apoptosis by activating downstream effector molecules.
Nuciferine inhibited the metastasis and migration of lung cancer cells by targeting sex determining region Y (SRY)-box 2 (SOX2), a transcription factor that positively associated with the drug-resistant property. Subsequently, nuciferine inactivated the AKT and STAT3 pathway, inhibited EMT while promoted mesenchymal-to-epithelial transition (MET) through down-regulating the expression of Slug and up-regulating the expression of N-cadherin and vimentin [2]. Nuciferine was also found to inhibit angiogenesis through downregulation of the expression of the CD133 (stemness marker), Bcl-2, hypoxia-inducible factor-1 ((HIF1α), an angiogenesis marker) and vascular endothelial growth factor A (VEGF-A) [2]. The co-treatment of isocorydine (0.4 mg/kg, IP) and gemcitabine also downregulated the expression of EMT-related transcription factors and STAT3, an upstream mediator of EMT, ultimately inhibiting metastasis, migration, and viability of breast cancer cells [106]. The analysis of the Traditional Chinese Medicine network pharmacology revealed to Qi et al. [96] that nuciferine (9.5 mg/mL, IP) works against human neuroblastoma and mouse colorectal cancer through inactivating of the PI3K-AKT signaling pathway and inhibiting the expression of IL-1β. Nuciferine (7.5 mg/kg, IP) inhibited p-AKT and p-ERK and subsequently down-regulated the downstream targets, including Nrf2, HIF-1α, P-gp, and BCRP, indicating a positive linkage with the multidrug resistance to paclitaxel [108].
Magnoflorine (10 mg/kg, IP) was found to be effectively promoting autophagy of diverse tumor cells through inactivating the PI3K/AKT/mTOR signaling and up-regulating autophagy proteins, including the p62, beclin-1 and LC3-II [110]. Besides, the co-treatment of magnoflorine (3 mg/kg, IP) and doxorubicin has been shown not only to reduce the cardiotoxicity of doxorubicin, but also to promote autophagy in tumor progression [20]. An aristolactam analogue named KO-202125 (0.078–1.95 mg/kg, intragastric administration) inhibited the activity of epidermal growth factor receptor (EGFR), a marker of poor prognosis in many types of cancer, and downregulation of the PI3K/AKT pathway as well as its downstream signals in breast cancer cells, such as glycogen synthase kinase 3β (GSK-3β) and p27 [111].
MAPK signaling pathway
MAPKs signaling pathway is one of the most important cellular mechanisms responsible for cell proliferation and mainly includes three subfamilies: ERK1/2, p38, and stress-activated protein kinases (JNK). As an ion channel protein overexpressed in various cancers, the R515, L522, and E624 amino acid residues of the transmembrane protein 16A (TMEM16A) proposed by molecular docking experiments was inhibited by nuciferine (30 μM, oral). And the phosphorylation of MEK1/2 and ERK1/2 (MAPK signaling) was also inhibited by nuciferine in LA795 Cells [2]. Additionally, the expression of p38 MAPK was markedly activated by the treatment of nuciferine, which induced cell cycle arrest (decreased cyclin dependent kinases 1/2 (CDK1/2) and cyclin B1, increased p53 and p21 expression), apoptosis (decreased Bcl-2 and increased cleaved caspase-9 and 3) and autophagy (increased beclin-1 and LC3) [20], [112].
In the gastric cancer mice model, magnoflorine (10 mg/kg, IP) remarkably activated JNK phosphorylation through the regulation of reactive oxygen species (ROS), while had no influence on p38 and ERK1/2, and further induced cell cycle arrest (increased p21 and p27, and decreased cyclin-A and cyclin-B1) and apoptosis (increased cleaved caspase-3 and cleaved poly-ADP-ribose polymerases), inhibiting human gastric cancer progression [110]. However, magnoflorine inhibted lipopolysaccharide-induced macrophages inflammatory responses via blocking the MAPK signaling pathways (attenuated the phosphorylation of p65, ERK, JNK, and p38). At present, the interaction between magnoflorine, macrophage and immune response is still not clear, whereas lipopolysaccharide-induced inflammation was indeed inhibited by the inactivation of the MAPK signaling pathways [113]. Furthermore, numerous studies demonstrated a crosstalk between various pathways, for example, both the phosphorylations of JNK and p38 were activated by boldine (90 mg/kg, oral) in a dose-dependent manner and maintain a dynamic balance. The inhibition of mouse mammary carcinoma by boldine was associated with the proapoptotic effect of JNK and p38 kinases and antiapoptotic action of ERK1/2 [114].
Wnt/β-catenin signaling
The abnormal regulation of the transcription factor β-catenin in the canonical Wnt signaling pathway induces diverse physiological processes such as cellular proliferation and differentiation. β-catenin regulates apoptotic proteins such as Bcl-2 and/or Bax and β-catenin is also regulated by a multiprotein complex, including Axin, adenomatous polyposis coli, and GSK-3β. Of note, several AAs perform their therapeutic effect by targeting the Wnt/β-catenin signaling in cancer. A previous work showed that nuciferine (50 mg/kg, IP) degraded β-catenin through Axin (a vital scaffold protein) accumulation and stabilization, and further suppresses Wnt/β-catenin signaling pathway through the downregulation of the targeted factors including c-myc, cyclin D and VEGF-A. In addition, it exerted pro-apoptosis effects through downregulating Bcl-2 and upregulating Bax, consequently resulting in the suppression of proliferation, metastasis, and growth of non-small cell lung cancer cells [97].
NF-κB signaling pathway
The NF-κB plays a well-known role in controlling inflammation, immunity, proliferation, and cell death, and growing evidence supports that NF-κB signaling pathway is activated in many cancers. The regulation of NF-κB signaling pathway is generally accomplished by the key protein p65, and TLR4, a member of the toll-like receptor family, assisting in the initiation of the innate immune response [115], [116]. As a hopeful natural product used for dietary adjuvant therapy, nuciferine (50 mg/kg, oral) inhibited melanoma cell growth by reducing p65 phosphorylation and downregulating TLR4 expression in a dose-dependent manner, ultimately inhibiting the NF-κB signaling pathway [117]. Meanwhile, good binding ability of magnoflorine with inflammation cytokines, like TNF-α, IL-6, IL-1β, and MCP-1, was identified by molecular docking analysis. The inactivation of NF-κB signaling induced by magnoflorine was also identified in rencent study [113].
Cell autophagy, apoptosis, and cycle arrest
Autophagy eliminates cytotoxic materials to exert cytoprotection through the lysosomal degradation pathway and plays an important role in limiting cancer initiation. Apoptosis is generally triggered by the intrinsic (mitochondrial) or the extrinsic (death receptor) pathway to induce cell death and guarantee normal cell turnover. The induction of cell cycle arrest is a valuable strategy in tumor inhibition, which can be regulated by CDKs and cyclins.
The cotreatment with magnoflorine and doxorubicin induced breast cell death in vivo by upregulating Beclin-1 and LC3-II and downregulating p62, ultimately leading to autophagosome formation. Additionally, this cotreatment inhibited cell proliferation by upregulating the expression of p53 and p21, resulting in the decreased expression of CDK1, CDK2, and cyclin B, ultimately activating the mitochondrial apoptosis pathway [20], [118]. Isocorydine (20 mg/kg, SC or 0.4 mg/kg, IP) inhibited hepatocellular carcinoma cell growth by upregulating the programmed cell death 4 (PDCD4, a tumor suppressor protein). Besides, isocorydine induced cell cycle arrest and intrinsic apoptosis through downregulating CDKs and antiapoptotic proteins, such as Bcl-2, Bcl-xl, and Mcl-1, and upregulating proapoptotic protein expression, such as Bax, Bim, Bik, and Bak [99], [119]. Indeed, the excellent anticancer activity of d-isocorydine (100–250 mg/kg, IP), highlights the significance of the C8 position on the basic skeleton of simple aporphines as a key modifying site, such as amino, carbonyl, and acetyl amino group, described in the section 5.2 [35], [100]. It suggests that further exploration and modification of this particular position may lead to the development of even more potent anticancer agents within the AA class.
The anti-cancer mechanism of action for many AAs involves up-regulating the apoptotic protease activating factor-1 (Apaf-1), caspase 9 and 3, as well as the poly ADP ribose polymerase (PARP, an apoptotic protein), all of which play significant roles in apoptosis and programmed tumor cell death. Many AAs exerted anticancer activities by regulating these factors, including boldine (50–100 mg/kg, IP or 90 mg/kg, intragastric administration) [94], [120], 7-Hydroxydehydronuciferine (20 mg/kg, IP) [121], apomorphine (50 μM) [19], and d-isocorydine (100–250 mg/kg, IP), as well as oxoisoaporphine complexes [104], [105]. Metal-alkaloid complexes, such as yttrium (III) and dysprosium (III) oxoglaucine, have been shown to inhibit the malignant proliferation of HepG2 cells through the degradation of Cdc25A and subsequent inactivation of CDK2 [95].
Additionally, the development of topoisomerase inhibitors represents a hopeful approach to targeting DNA therapy. A chiral platinum complex derivative of oxoaporphine successfully inhibited the growth of HepG2 cells by interacting with p53 binding protein 1 (53BP1), TRF1, c-myc, TRF2 and human telomerase reverse transcriptase (hTERT), targeting DNA and suppressing topoisomerase I activity, leading to S-phase arrest and apoptosis via the mitochondrion-mediated pathway [105]. In addition, the simple aporphines, such as isocorydine [99], magnoflorine [118], xylopine [122], dicentrine [123], boldine and its derivates [124], [125], and an oxoAA liriodenine, [25] inhibited DNA topoisomerases, and induced cell cycle arrest and apoptosis through modulating hTERT, p53, Bax, Bcl-2 and caspase 3, 9, 8 with intrinsic and extrinsic pathways to limit tumor progression. Oxocrebanine, a simple aporphine isolated from Stephania hainanensis, was a novel dual topoisomerase inhibitor of Topo I and IIα. Oxocrebanine also induced autophagy by increasing the LC3-II/I ratio, Beclin-1 expression and autophagosome formation. It is a dual topoisomerase inhibitor with potential therapeutic applications [126]. Besides, oxostephanine played dual roles in inhibiting aurora kinase activity and angiogenesis [127]. Both docking and molecular dynamics simulations indicated that boldine and the derivative (N-benzylsecoboldine hydrochloride) [124], 4,5-dehydro-9-methoxyguatterfriesine [128], guadiscine and guadiscidine [129] inhibited telomerase activity by regulating the transcription of the catalytic subunit, telomerase reverse transcriptase.
Notably, the inhibition ability of topoisomerases is tightly associated with the planar conformations of compounds. Qing et al. [23] proposed that the conjugation and planar conformation of simple aporphine ring system should be extended by dihydroxy- and oxo- groups, enabling dihydroxyaporphine and oxoAAs to easily intercalate into DNA and inhibiting topoisomerases. Besides, certain substitution groups, namely 1,2-methylenedioxy, N-methyl, and N-2-hydroxypropyl groups, were found to be the crucial factor in enhancing the anticancer activity of AAs. Conversely, the compounds with a ruptured C-ring and a lengthy N-chain, or those substituted by hydroxyl or methyl group at the C7 position, and methyl or acetyl group at nitrogen, as well as quaternary nitrogen on their skeletons, had an adverse effect [23].
Regulation of gut microbiota
Preclinical and clinical evidence suggests that gut microbiota is an important regulator of cancer development, progression, metastasis and treatment response [130]. Accordingly, the microbiota is gaining increasing attention in cancer diagnosis and treatment. Oral treatment of berberine improved brain dopa/dopamine levels through interactions with the gut microbiota, leading to the amelioration of Parkinson's disease symptoms in patients with hyperlipidemia. The fecal transplantation studies in Parkinson's disease mice have also revealed that berberine improved the disease by activating the gut-brain axis [131]. At the genus level, nuciferine improved colitis by reducing the abundance of Bilophila and Halomonas in vivo, which were regared as harmful gut microbes, indicating that nuciferine exerted anti-inflammatory activity through regulating the gut microbiota [21]. At present, lack of literature investigating the specific role of gut microbiota in relation to the anti-cancer activities of AAs. There are tremendous opportunities to elucidate the causal and molecular interactions between gut microbiota and AAs in the context of cancer.
Other signaling pathways
AMPK is considered as the regulator of metabolism, modulating ATP synthesis and consumption to balance body energy level. Nuciferine shows promise as an adjunctive drug to gemcitabine for treating pancreatic cancer by activating AMPK, which subsequently down-regulates HMGCR and YAP. The latter is a major regulator of the mevalonate pathway and an overexpressed protein that contributes to pancreatic cancer progression. Nuciferine (30 mg/kg, IP) showed promise as an adjuvant drug to gemcitabine for treating pancreatic cancer treatment by activating AMPK, which subsequently down-regulated HMGCR and YAP, a major regulator of the mevalonate pathway and an overexpressed protein in pancreatic cancer progression, respectively [107].
Besides, apomorphine was used as a pre-metastatic drug to treat primary tumors before they spread to the brain because of its ability to inhibit tumor cell migration. [102]. The results showed that brain metastasis initiating cells (BMICs) possessed a distinct genetic profile. and apomorphine's ability to cross the BBB has demonstrated its efficacy in targeting BMICs, with the potential to target neural development systems or related disorders at low IC50. Besides, apomorphine (5 mg/kg, SC) greatly attenuated brain metastasis development in apomorphine-treated brains both in silico and in vivo through the downregulation of 3 genes, KIF16B, SEPW1, and TESK2 [102]. Protein-protein interactions were identified as novel therapeutic targets. A simple aporphine dicentrine emerged as the be most effective docked alkaloid against breast cancer by regulating tumor suppressor protein p53 and breast cancer associated protein [132].
Approximately 50 AAs exerted anticancer effects in different cancer types. Among them, isocorydine, nuciferine, boldine, magnoflorine, and their derivates exhibit pharmacological activities in several cancers, providing a theoretical basis for further in-depth experiment and clinical trials. These AAs mainly inhibited cell proliferation and migration, and induce apoptosis through the PI3K-AKT, MAPK, Wnt/β-catenin, NF-κB pathways to induce cell autophagy, apoptosis, and cell cycle arrest. However, mechanisms involving the AA treatment with microbial are still largely unknown. According to the structure–activity relationship, the strong activity of oxoaporphines, dehydroaporphines and other AAs are attributed to their stable conjugation and planar conformation and special substitution groups (1,2-methylenedioxy, N-methyl and N-2-hydroxypropyl group), which allow their easy intercalation into DNA and inhibit topoisomerases. However, lack of supportin g data from in vivo models and clinical trials confirmed the antitumor activity and its mechanisms.
Conclusion
There is an increased interest in the development of AA drugs targeting various diseases especially cancers. The studies of administration of the AAs in vivo show their characteristics, such as fast absorption, poor bioavailability as well as wide tissue distribution, which can be attributed to their small molecular weight, nonpolar hydrocarbon backbones and hydrophobic functional groups like aromatic rings. However, poor oral bioavailability is a major obstacle for the development of AAs. The toxicities of AAs are tightly linked with the dosage and administration ways. Side effects, such as subcutaneous nodules, nausea, and somnolence, are reported by apomorphine treatment with 2–6 mg/kg subcutaneous infusion administration in Parkinson's disease patients, whereas most AAs shows no toxicity with specific models. Strategies on bioavailability and systemic toxicity improvement have been reported, such as changing the ways of administration, developing delivery systems (nanoparticles, liposomes), and co-administration with absorption enhancers [133]. Recently, the effects of metabolic enzymes and gut microbiota on AA metabolism have been identified, therefore, the improvement of AA absorption, especially their metabolism in intestine, would be an important direction to be explored.
Undoubtedly, AAs will continue to attract the attention of scientists due to their potential anticancer activity and demand of alternative chemotherapeutic drugs. Different AAs showed anticancer activities in specific tumor models. Thus, it is essential to fully evaluate the safety, pharmacokinetics, and molecular mechanisms of these AAs. Novel chemical structures and the structure–activity relationship based on the high structural diversity of AAs need to be more explored as well. In particular, new strategies should be established to improve AA bioavailability, ultimately enhancing their therapeutic benefits. Altogether, AAs remain a promising pool for the discovery of various bioactivities that could be effectively further developed in cancer therapy based on clinical studies.
CRediT authorship contribution statement
Jing Sun: Investigation, Writing – original draft. Xingtian Zhan: Writing – review & editing, Supervision. Weimin Wang: Supervision, Writing – review & editing. Xiaojie Yang: Investigation, Writing – review & editing. Yichen Liu: Writing – original draft. Huanzhi Yang: Investigation, Resources. Jianjun Deng: Conceptualization, Supervision. Haixia Yang: Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research is supported by National Key Research and Development Program (2022YFF1100205), the National Natural Science Foundation of China (21978229 and 21676212) and Chinese Universities Scientific Fund (15053342).
Biographies
Jing SUN received her master degree from Northwest University of Food Science and Technology in 2022. Now she is a doctoral student in China Agricultural University of Food Science and Nutrition Engineering. Currently, her research primarily focuses on the hepatoprotective effects of natural products.
Xingtian Zhan obtained his bachelor degree from Southeast University of biological engineering in 2015, now he is a doctoral student majoring in Social Medicine and Health Management in Renmin University of China. Currently his research primarily focuses on the health economics and health policy.
Weimin WANG obtained his bachelor degree of Food Science and Engineering from Hefei University of Technology in 2022, now he is a master candidate of China Agricultural University. Currently his research primarily focuses on the nutrition and chronic disease prevention.
Xiaojie YANG received her bachelor degree from Jiangnan University of Food Science and Engineering in 2022, now she is a master candidate of China Agricultural University. Currently her research primarily focuses on the nutrition and chronic disease prevention.
Yichen LIU obtained her bachelor degree from South-Central Minzu University of Food Quality and Safety in 2022, now she is a master candidate of China Agricultural University. Her research primarily focuses on the analysis of the nutritional composition of Lycium barbarum seeds and the effect of UHP on their polyphenols.
Huanzhi YANG graduated from Hunan Agricultural University in 2018 with a master's degree in bioengineering. In 2019, he studied at the College of Food Science and Nutrition Engineering of China Agricultural University as a doctoral student in Food Science.
Jianjun DENG obtained his PhD in food biotechnology from China Agricultural University in 2010. He completed his post-doctoral training at the University of Hong Kong and Harvard Medical School. He is currently a professor of School of Chemical Engineering in Northwest University. His research interests include natural products and chronic disease prevention and control, food chemistry and nutrition, fatty acid metabolism and cancer. He has published more than 130 academic papers and is currently an associate editor of eFood and a guest editor of Nutrients and Antioxidants.
Haixia YANG received her PhD in Food Science College from China Agricultural University in 2011. She studied as a postdoc research fellow in University of Massachusetts Amherst and Harvard Medical School. Now she is a professor working at the College of Food Science and Nutritional Engineering from China Agricultural University. She has published more than 100 papers related to the food science, including protein purification and function, phytochemicals and inflammation-associated diseases and cancer. She is currently an editor of Frontiers in Nutrition, Nutrients, Antioxidants, and Protein & Peptide Letters.
Contributor Information
Xingtian Zhan, Email: ruczhanxt@163.com.
Haixia Yang, Email: hyang@cau.edu.cn.
References
- 1.Xia C., Dong X., Li H., Cao M., Sun D., He S., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5) doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai X., Liu X.Y., Li S.T., An H.L., Kang X.J., Guo S. Nuciferine inhibits TMEM16A in dietary adjuvant therapy for lung cancer. J Agric Food Chem. 2022;70(12):3687–3696. doi: 10.1021/acs.jafc.1c08375. [DOI] [PubMed] [Google Scholar]
- 3.Byrne S., Boyle T., Ahmed M., Lee S.H., Benyamin B., Hyppönen E. Lifestyle, genetic risk and incidence of cancer: a prospective cohort study of 13 cancer types. Int J Epidemiol. 2023;52(3):817–826. doi: 10.1093/ije/dyac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Z., Yang H., Zhu C., Fan D., Deng J. Dietary phytochemicals: As a potential natural source for treatment of Alzheimer's Disease. Food Innovation and Advances. 2023;2(1):36–43. doi: 10.48130/FIA-2023-0007. [DOI] [Google Scholar]
- 5.Yang H., Xiao L., Yuan Y., Luo X., Jiang M., Ni J., et al. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem Pharmacol. 2014;92(4):599–606. doi: 10.1016/j.bcp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Fan W., Zong H., Zhao T., Deng J., Yang H. Bioactivities and mechanisms of dietary proanthocyanidins on blood pressure lowering: A critical review of in vivo and clinical studies. Crit Rev Food Sci Nutr. 2022;1549–7852 doi: 10.1080/10408398.2022.2132375. [DOI] [PubMed] [Google Scholar]
- 7.Atanasov A.G., Zotchev S.B., Dirsch V.M., Orhan I.E., Banach M., Rollinger J.M., et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Li D., Tian Q., Ding Y., Jiang H., Xin G., et al. The effect of kiwi berry (Actinidia arguta) on preventing and alleviating loperamide-induced constipation. Food Innovation and Advances. 2023;2(1):1–8. doi: 10.48130/FIA-2023-0001. [DOI] [Google Scholar]
- 9.Yang H., Rothenberger E., Zhao T., Fan W., Kelly A., Attaya A., et al. Regulation of inflammation in cancer by dietary eicosanoids. Pharmacol Ther. 2023;248 doi: 10.1016/j.pharmthera.2023.108455. [DOI] [PubMed] [Google Scholar]
- 10.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 11.Sharifi-Rad JA-O, Quispe C, Patra JK, Singh YD, Panda MK, Das G, et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxid Med Cell Longev. 2021(1942-0994 (Electronic)). doi: https://doi.org/10.1155/2021/3687700. [DOI] [PMC free article] [PubMed]
- 12.Smith E.R., Wang J., Yang D., Xu X. Paclitaxel resistance related to nuclear envelope structural sturdiness. Drug Resist Updat. 2022;65 doi: 10.1016/j.drup.2022.100881. [DOI] [PubMed] [Google Scholar]
- 13.Dhyani P., Quispe C., Sharma E., Bahukhandi A., Sati P., Attri D.C., et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22(1):206. doi: 10.1186/s12935-022-02624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yx W. Chemical Industry Press Co., Ltd.; Beijing: 2005. Alkaloids: A Handbook of Practical Natural Products. Alkaloids. [Google Scholar]
- 15.Silva T.R.C., Rita B.H., Raminelli C. Advances Towards the Synthesis of Aporphine Alkaloids: C-Ring Formation via Approaches Based on One- and Two-Bond Disconnections. Chem Rec. 2022;22(2):e202100246. doi: 10.1002/tcr.202100246. [DOI] [PubMed] [Google Scholar]
- 16.Shang X.F., Yang C.J., MorrisNatschke S.L., Li J.C., Yin X.D., Liu Y.Q., et al. Biologically active isoquinoline alkaloids covering 2014–2018. Med Res Rev. 2020;40(6):2212–2289. doi: 10.1002/med.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F., Zhu N., Zhou F., Lin D. Natural aporphine alkaloids with potential to impact metabolic syndrome. Molecules. 2021;26(20) doi: 10.3390/molecules26206117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng H., Han L., Shi W., Fang X., Hong Y., Cao Y. Research Advances in Lotus Leaf as Chinese Dietary Herbal Medicine. Am J Chin Med. 2022;50(06):1423–1445. doi: 10.1142/S0192415X22500616. [DOI] [PubMed] [Google Scholar]
- 19.Lee J., Ham J., Lim W., Song G. Apomorphine facilitates loss of respiratory chain activity in human epithelial ovarian cancer and inhibits angiogenesis in vivo. Free Radic Biol Med. 2020;154:95–104. doi: 10.1016/j.freeradbiomed.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Tian W., Xie X., Cao P. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed Pharmacother. 2020:121. doi: 10.1016/j.biopha.2019.109139. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y., Zhao Q., Huang Q., Li Y., Yu J., Zhang R., et al. Nuciferine Regulates Immune Function and Gut Microbiota in DSS-Induced Ulcerative Colitis. Front Vet Sci. 2022:9. doi: 10.3389/fvets.2022.939377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yq Q.u., Qy Z., Xy T., Zheng G.h., Zp Q., Xx T. Effect of nuciferine against the proliferation of cholangiocarcinoma cells through Akt/mTOR/4EBP1-glycolytic pathway. Nat Prod Res Dev. 2023;35(8):1297–1304. [Google Scholar]
- 23.Qing Z.X., Huang J.L., Yang X.Y., Liu J.H., Cao H.L., Xiang F., et al. Anticancer and reversing multidrug resistance activities of natural isoquinoline alkaloids and their structure-activity relationship. Curr Med Chem. 2018;25(38):5088–5114. doi: 10.2174/0929867324666170920125135. [DOI] [PubMed] [Google Scholar]
- 24.Karki A., Namballa H.K., Alberts I., Harding W.W. Structural manipulation of aporphines via C10 nitrogenation leads to the identification of new 5-HT7AR ligands. Bioorg Med Chem. 2020;28(15) doi: 10.1016/j.bmc.2020.115578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordin N., Majid N.A., Hashim N.M., Rahman M.A., Hassan Z., Ali H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des Devel Ther. 2015;9:1437–1448. doi: 10.2147/DDDT.S77727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y.B., Li Y.X., Song H., Feng X.J. Design, synthesis and anticancer activity of oxoaporphine alkaloid derivatives. J Enzyme Inhib Med Chem. 2014;29(5):722–727. doi: 10.3109/14756366.2013.845818. [DOI] [PubMed] [Google Scholar]
- 27.Honda T., Shigehisa H. Novel and efficient synthetic path to proaporphine alkaloids: Total synthesis of (+/-)-stepharine and (+/-)-pronuciferine. Org Lett. 2006;8(4):657–659. doi: 10.1021/ol052841m. [DOI] [PubMed] [Google Scholar]
- 28.Latolla N., Hlangothi B. Three new proaporphine alkaloids from Cissampelos capensis L.f. and their cytotoxic evaluation. Nat Prod Res. 2022 doi: 10.1080/14786419.2022.2146687. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V., Poonam P.AK., Parmar V.S. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat Prod Rep. 2003;20(6):565–583. doi: 10.1039/b303648k. [DOI] [PubMed] [Google Scholar]
- 30.Lin C.H., Ko F.N., Wu Y.C., Lu S.T., Teng C.M. The relaxant actions on guinea-pig trachealis of atherosperminine isolated from fissistigma-glaucescens. Eur J Pharmacol. 1993;237(1):109–116. doi: 10.1016/0014-2999(93)90099-4. [DOI] [PubMed] [Google Scholar]
- 31.Nasrullah A.A., Zahari A., Mohamad J., Awang K. Antiplasmodial alkaloids from the bark of cryptocarya nigra (lauraceae) Molecules. 2013;18(7):8009–8017. doi: 10.3390/molecules18078009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bin Nadzirin I., Fortuny-Gomez A., Ngum N., Richards D., Ali S., Searcey M., et al. Taspine is a natural product that suppresses P2X4 receptor activity via phosphoinositide 3-kinase inhibition. Br J Pharmacol. 2021;178(24):4859–4872. doi: 10.1111/bph.15663. [DOI] [PubMed] [Google Scholar]
- 33.Ali G., Cuny G.D. 8-, 9-, and 11-aryloxy dimeric aporphines and their pharmacological activities. Molecules. 2021;26(15) doi: 10.3390/molecules26154521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morikawa T., Kitagawa N., Tanabe G., Ninomiya K., Okugawa S., Motai C., et al. Quantitative determination of alkaloids in lotus flower (flower buds of nelumbo nucifera) and their melanogenesis inhibitory activity. Molecules. 2016;21(7) doi: 10.3390/molecules21070930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong M., Liu Y.J., Liu J.X., Di D.L., Xu M.R., Yang Y.Y., et al. Isocorydine derivatives and their anticancer activities. Molecules. 2014;19(8):12099–12115. doi: 10.3390/molecules190812099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D.H., Li J.Y., Xue C.M., Han T., Sai C.M., Wang K.B., et al. Antiproliferative dimeric aporphinoid alkaloids from the roots of thalictrum cultratum. J Nat Prod. 2017;80(11):2893–2904. doi: 10.1021/acs.jnatprod.7b00387. [DOI] [PubMed] [Google Scholar]
- 37.Singh H., Singh D., Lekhak M.M. Ethnobotany, botany, phytochemistry and ethnopharmacology of the genus Thalictrum L. (Ranunculaceae): A review. J Ethnopharmacol. 2023:305. doi: 10.1016/j.jep.2022.115950. [DOI] [PubMed] [Google Scholar]
- 38.Liu C., Kao C., Wu H., Li W., Huang C., Li H., et al. Antioxidant and anticancer aporphine alkaloids from the leaves of nelumbo nucifera gaertn. Cv Rosa-plena Molecules. 2014;19(11):17829–17838. doi: 10.3390/molecules191117829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohamed S.M., Hassan E.M., Ibrahim N.A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat Prod Res. 2010;24(15):1395–1402. doi: 10.1080/14786410902906959. [DOI] [PubMed] [Google Scholar]
- 40.Min Y.D., Choi S.U., Lee K.R. Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of Sinomenium acutum. Arch Pharm Res. 2006;29(8):627–632. doi: 10.1007/BF02968246. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., He G., Li H., He J., Feng E., Bai L., et al. Plasma pharmacokinetics and tissue distribution study of roemerine in rats by liquid chromatography with tandem mass spectrometry (LC-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:249–255. doi: 10.1016/j.jchromb.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura S., Nakashima S., Tanabe G., Oda Y., Yokota N., Fujimoto K., et al. Alkaloid constituents from flower buds and leaves of sacred lotus (Nelumbo nucifera, Nymphaeaceae) with melanogenesis inhibitory activity in B16 melanoma cells. Bioorg Med Chem. 2013;21(3):779–787. doi: 10.1016/j.bmc.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 43.Fu L., Dai D.C., Yang R., Chen G.Y., Zheng C.J., Song X.M., et al. Two novel aporphine-derived alkaloids from the stems of Fissistigma glaucescens. Fitoterapia. 2021:155. doi: 10.1016/j.fitote.2021.105036. [DOI] [PubMed] [Google Scholar]
- 44.Li D. Natural deep eutectic solvents in phytonutrient extraction and other applications. Front. Plant Sci. 2022:13. doi: 10.3389/fpls.2022.1004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strzemski M., Dresler S., Podkościelna B., Skic K., Sowa I., Załuski D., et al. Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids. Molecules. 2022 doi: 10.3390/molecules27092815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres-Vega J., Gomez-Alonso S., Perez-Navarro J., Pastene-Navarrete E. Green extraction of alkaloids and polyphenols from peumus boldus leaves with natural deep eutectic solvents and profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants (Basel) 2020;9(2) doi: 10.3390/plants9020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takla S.S., Shawky E., Hammoda H.M., Darwish F.A. Green techniques in comparison to conventional ones in the extraction of Amaryllidaceae alkaloids: Best solvents selection and parameters optimization. J Chromatogr A. 2018;1567:99–110. doi: 10.1016/j.chroma.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Uwineza P.A., Waśkiewicz A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules. 2020 doi: 10.3390/molecules25173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arumugham T, K R, Hasan SW, Show PL, Rinklebe J, Banat F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications – A review. Chemosphere. 2021;271:129525. doi: https://doi.org/10.1016/j.chemosphere.2020.129525. [DOI] [PubMed]
- 50.Hu C., Gan X., Jia Q., Gao P., Du T., Zhang F. Optimization of supercritical-CO2 extraction and pharmacokinetics in SD rats of alkaloids form Sophora moorcroftiana seed. Sci Rep. 2022;12(1):3301. doi: 10.1038/s41598-022-07278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Djapic N. Supercritical Carbon Dioxide Extraction of Nicotiana tabacum Leaves: Optimization of Extraction Yield and Nicotine Content. Molecules. 2022 doi: 10.3390/molecules27238328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atwi-Ghaddar S., Zerwette L., Destandau E., Lesellier E. Supercritical Fluid Extraction (SFE) of Polar Compounds from Camellia sinensis Leaves: Use of Ethanol/Water as a Green Polarity Modifier. Molecules. 2023 doi: 10.3390/molecules28145485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gan R., Liu Y., Li H., Xia Y., Guo H., Geng F., et al. Natural sources, refined extraction, biosynthesis, metabolism, and bioactivities of dietary polymethoxyflavones (PMFs) Food Sci Hum Wellness. 2024;13(1):27–49. doi: 10.26599/FSHW.2022.9250003. [DOI] [Google Scholar]
- 54.Liu Y., Liu H.-Y., Li S.-H., Ma W., Wu D.-T., Li H.-B., et al. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: An updated review. TrAC Trends Anal Chem. 2022;149 doi: 10.1016/j.trac.2022.116554. [DOI] [Google Scholar]
- 55.He Q., Lei Q., Huang S., Zhou Y., Liu Y., Zhou S., et al. Effective extraction of bioactive alkaloids from the roots of Stephania tetrandra by deep eutectic solvents-based ultrasound-assisted extraction. J Chromatogr A. 2023;1689 doi: 10.1016/j.chroma.2022.463746. [DOI] [PubMed] [Google Scholar]
- 56.Ma W., Lu Y., Hu R., Chen J., Zhang Z., Pan Y. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta. 2010;80(3):1292–1297. doi: 10.1016/j.talanta.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 57.Ruan Y.Q., Xu J.H., Chu J.B., Shi J., Shi Q.Y. Processing tactics for low-cost production of pure nuciferine from lotus leaf. Ultrason Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal Chim Acta. 2011;703(1):8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 59.Okiyama D.C.G., Soares I.D., Cuevas M.S., Crevelin E.J., Moraes L.A.B., Melo M.P., et al. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res Int. 2018;114:20–29. doi: 10.1016/j.foodres.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 60.Agbo F., Isaacson S.H., Gil R., Chiu Y.Y., Brantley S.J., Bhargava P., et al. Pharmacokinetics and comparative bioavailability of apomorphine sublingual film and subcutaneous apomorphine formulations in patients with parkinson's disease and “off'' episodes: Results of a randomized, three-way crossover, open-label study. Neurol Ther. 2021;10(2):693–709. doi: 10.1007/s40120-021-00251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z., Liu J., Li Y., Du X., Li Y., Wang R., et al. Identify super quality markers from prototype-based pharmacokinetic markers of Tangzhiqing tablet (TZQ) based on in vitro dissolution/ permeation and in vivo absorption correlations. Phytomedicine. 2018;45:59–67. doi: 10.1016/j.phymed.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Gu S.Y., Zhu G.H., Wang Y.Z., Li Q., Wu X., Zhang J.G., et al. A sensitive liquid chromatography-tandem mass spectrometry method for pharmacokinetics and tissue distribution of nuciferine in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;961:20–28. doi: 10.1016/j.jchromb.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 63.Wang F.G., Cao J., Hou X.Q., Li Z.Y., Qu X.L. Pharmacokinetics, tissue distribution, bioavailability, and excretion of nuciferine, an alkaloid from lotus, in rats by LC/MS/MS. Drug Dev Ind Pharm. 2018;44(9):1557–1562. doi: 10.1080/03639045.2018.1483399. [DOI] [PubMed] [Google Scholar]
- 64.Zou S., Ge Y., Chen X., Li J., Yang X., Wang H., et al. Simultaneous determination of five alkaloids by HPLC-MS/MS combined with micro-SPE in rat plasma and its application to pharmacokinetics after oral administration of lotus leaf extract. Front Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang X., Hao N., Chen G., Liu S., Che Z. Chemistry and biology of nuciferine. Ind Crops Prod. 2022;179 doi: 10.1016/j.indcrop.2022.114694. [DOI] [Google Scholar]
- 66.Ye L.H., He X.X., You C., Tao X., Wang L.S., Zhang M.D., et al. Pharmacokinetics of nuciferine and n-nornuciferine, two major alkaloids from nelumbo nucifera leaves, in rat plasma and the brain. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y., Wu X., Mi Y., Zhang B., Gu S., Liu G., et al. PLGA nanoparticles for the oral delivery of nuciferine: preparation, physicochemical characterization and in vitro/in vivo studies. Drug Deliv. 2017;24(1):443–451. doi: 10.1080/10717544.2016.1261381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue B.J., Zhao Y.Y., Miao Q., Miao P.P., Yang X.Y., Sun G.X., et al. In vitro and in vivo identification of metabolites of magnoflorine by LC LTQ-Orbitrap MS and its potential pharmacokinetic interaction in Coptidis Rhizoma decoction in rat. Biomed Chromatogr. 2015;29(8):1235–1248. doi: 10.1002/bmc.3413. [DOI] [PubMed] [Google Scholar]
- 69.Tian X., Li Z., Lin Y., Chen M., Pan G., Huang C. Study on the PK profiles of magnoflorine and its potential interaction in Cortex phellodendri decoction by LC-MS/MS. Anal Bioanal Chem. 2014;406(3):841–849. doi: 10.1007/s00216-013-7530-9. [DOI] [PubMed] [Google Scholar]
- 70.Guo C.C., Yu C.H., Li L., Wang Y.Q., Wang S.J., Wang W.H., et al. Rapid determination of isocorydine in rat plasma and tissues using liquid chromatography - tandem mass spectrometry and its applications to pharmacokinetics and tissue distribution. Xenobiotica. 2012;42(5):466–476. doi: 10.3109/00498254.2011.640965. [DOI] [PubMed] [Google Scholar]
- 71.Wei J.X., Yu Y.Y., Li Y.N., Shao J., Li J.Y., Li L.Z., et al. Pharmacokinetics, tissue distribution and excretion of 6-O-demethylmenisporphine, a bioactive oxoisoaporphine alkaloid from Menispermi Rhizoma, as determined by a HPLC-MS/MS method. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1156 doi: 10.1016/j.jchromb.2020.122297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bao S.H., Geng P.W., Wang S.H., Zhou Y.F., Hu L.F., Yang X.Z. Pharmacokinetics in rats and tissue distribution in mouse of magnoflorine by ultra performance liquid chromatography-tandem mass spectrometry. Int J Clin Exp Med. 2015;8(11):20168–20177. [PMC free article] [PubMed] [Google Scholar]
- 73.Lu W., He L.C., Zeng X.M. HPLC method for the pharmacokinetics and tissue distribution of taspine solution and taspine liposome after intravenous administrations to mice. J Pharm Biomed Anal. 2008;46(1):170–176. doi: 10.1016/j.jpba.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Tan J.P.K., Voo Z.X., Lim S., Venkataraman S., Ng K.M., Gao S., et al. Effective encapsulation of apomorphine into biodegradable polymeric nanoparticles through a reversible chemical bond for delivery across the blood–brain barrier. Nanomedicine. 2019;17:236–245. doi: 10.1016/j.nano.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 75.Li B.J., Han L.M., Cao B.Y., Yang X.Y., Zhu X.H., Yang B., et al. Use of magnoflorine-phospholipid complex to permeate blood-brain barrier and treat depression in the CUMS animal model. Drug Deliv. 2019;26(1):566–574. doi: 10.1080/10717544.2019.1616236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neef C., van Laar T. Pharmacokinetic-pharmacodynamic relationships of apomorphine in patients with Parkinson's disease. Clin Pharmacokinet. 1999;37(3):257–271. doi: 10.2165/00003088-199937030-00004. [DOI] [PubMed] [Google Scholar]
- 77.Alolga R.N., Fan Y., Zhang G., Li J., Zhao Y.J., Kakila J.L., et al. Pharmacokinetics of a multicomponent herbal preparation in healthy Chinese and African volunteers. Sci Rep. 2015;5 doi: 10.1038/srep12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao H., Zhang L., Zhu A., Liu X.Y., Wang T.X., Wan M.Q., et al. Metabolic profiling of nuciferine in vivo and in vitro. J Agric Food Chem. 2020;68(48):14135–14147. doi: 10.1021/acs.jafc.0c04468. [DOI] [PubMed] [Google Scholar]
- 79.Wu X.-L., Wu M.-J., Chen X.-Z., Ma H.-L., Ding L.-Q., Qiu F., et al. Metabolic profiling of nuciferine in rat urine, plasma, bile and feces after oral administration using ultra-high performance liquid chromatography-diode array detection-quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2017;140:71–80. doi: 10.1016/j.jpba.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Ye L.H., He X.X., Kong L.T., Liao Y.H., Pan R.L., Xiao B.X., et al. Identification and characterization of potent CYP2D6 inhibitors in lotus leaves. J Ethnopharmacol. 2014;153(1):190–196. doi: 10.1016/j.jep.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 81.Tang Z., Luo T., Huang P., Luo M., Zhu J., Wang X., et al. Nuciferine administration in C57BL/6J mice with gestational diabetes mellitus induced by a high-fat diet: the improvement of glycolipid disorders and intestinal dysbacteriosis. Food Funct. 2021;12(22):11174–11189. doi: 10.1039/D1FO02714J. [DOI] [PubMed] [Google Scholar]
- 82.Lai Y., Kuo T., Chen C., Tsai H., Lee S. Metabolism of dicentrine: Identification of the phase I and phase II metabolites in miniature pig urine. Drug Metab Dispos. 2010;38(10):1714–1722. doi: 10.1124/dmd.110.033795. [DOI] [PubMed] [Google Scholar]
- 83.Hroch M., Micuda S., Cermanova J., Chladek J., Tomsik P. Development of an HPLC fluorescence method for determination of boldine in plasma, bile and urine of rats and identification of its major metabolites by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;936:48–56. doi: 10.1016/j.jchromb.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Chen J.Z., Chou G.X., Wang C.H., Yang L., Bligh S.W.A., Wang Z.T. Characterization of new metabolites from in vivo biotransformation of norisoboldine by liquid chromatography/mass spectrometry and NMR spectroscopy. J Pharm Biomed Anal. 2010;52(5):687–693. doi: 10.1016/j.jpba.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Li Y., Zeng R.J., Chen J.Z., Wu Y.B., Chou G.X., Gao Y., et al. Pharmacokinetics and metabolism study of isoboldine, a major bioactive component from Radix Linderae in male rats by UPLC-MS/MS. J Ethnopharmacol. 2015;171:154–160. doi: 10.1016/j.jep.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 86.Tan Y.F., Wang R.Q., Wang W.T., Wu Y., Ma N., Lu W.Y., et al. Study on the pharmacokinetics, tissue distribution and excretion of laurolitsine from Litsea glutinosa in Sprague-Dawley rats. Pharm Biol. 2021;59(1):884–892. doi: 10.1080/13880209.2021.1944221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu W.N., McKown L.A. The in vitro metabolism of thalicarpine, an aporphine-benzyltetrahydroisoquinoline alkaloid, in the rat API-MS/MS identification of thalicarpine and metabolites. J Pharm Biomed Anal. 2002;30(1):141–150. doi: 10.1016/S0731-7085(02)00202-9. [DOI] [PubMed] [Google Scholar]
- 88.Liu L.Y., Zhou L., Liu X.Z., Zou D.J. Effect of hedan tablets on body weight and insulin resistance in patients with metabolic syndrome. Obes Facts. 2022;15(2):180–185. doi: 10.1159/000520711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaudhuri K.R., Leta V. Apomorphine infusion for improving sleep in Parkinson's disease. Lancet Neurol. 2022;21(5):395–398. doi: 10.1016/S1474-4422(22)00128-4. [DOI] [PubMed] [Google Scholar]
- 90.Dewey R.B., Hutton J.T., LeWitt P.A., Factor S.A. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for Parkinsonian off-state events. Arch Neurol. 2001;58(9):1385–1392. doi: 10.1001/archneur.58.9.1385. [DOI] [PubMed] [Google Scholar]
- 91.Esmaeili S., Shahidi G., Hajiakhondi F. Apomorphine induced immune hemolytic anemia. J Neurol Sci. 2019;405 doi: 10.1016/j.jns.2019.10.1274. [DOI] [Google Scholar]
- 92.Szalak R., Matysek M., Koval M., Dziedzic M., Kowalczuk-Vasilev E., Kruk-Slomka M., et al. Magnoflorine from berberis vulgaris roots-impact on hippocampal neurons in mice after short-term exposure. Int J Mol Sci. 2023;24(8) doi: 10.3390/ijms24087166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding C., Dai Z., Yu H., Zhao X., Luo X. New aporphine alkaloids with selective cytotoxicity against glioma stem cells from Thalictrum foetidum. Chin J Nat Med. 2019;17(9):698–706. doi: 10.1016/S1875-5364(19)30084-6. [DOI] [PubMed] [Google Scholar]
- 94.Subramaniam N., Kannan P., Ashokkumar K., Thiruvengadam D. Hepatoprotective effect of boldine against diethylnitrosamine-induced hepatocarcinogenesis in wistar rats. J Biochem Mol Toxicol. 2019;33(12) doi: 10.1002/jbt.22404. [DOI] [PubMed] [Google Scholar]
- 95.Wei J.H., Chen Z.F., Qin J.L., Liu Y.C., Li Z.Q., Khan T.M., et al. Water-soluble oxoglaucine-Y(III), Dy(III) complexes: in vitro and in vivo anticancer activities by triggering DNA damage, leading to S phase arrest and apoptosis. Dalton Trans. 2015;44(25):11408–11419. doi: 10.1039/c5dt00926j. [DOI] [PubMed] [Google Scholar]
- 96.Qi Q., Li R., Li H.Y., Cao Y.B., Bai M., Fan X.J., et al. Identification of the anti-tumor activity and mechanisms of nuciferine through a network pharmacology approach. Acta Pharmacol Sin. 2016;37(7):963–972. doi: 10.1038/aps.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu W., Yi D.D., Guo J.L., Xiang Z.X., Deng L.F., He L. Nuciferine, extracted from Nelumbo nucifera Gaertn, inhibits tumor-promoting effect of nicotine involving Wnt/beta-catenin signaling in non-small cell lung cancer. J Ethnopharmacol. 2015;165:83–93. doi: 10.1016/j.jep.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 98.Li Q.H., Sui L.P., Zhao Y.H., Chen B.G., Li J., Ma Z.H., et al. Tripartite motif-containing 44 is involved in the tumorigenesis of laryngeal squamous cell carcinoma, and its expression is downregulated by nuciferine. Tohoku J Exp Med. 2021;254(1):17–23. doi: 10.1620/tjem.254.17. [DOI] [PubMed] [Google Scholar]
- 99.Lu P., Sun H.F., Zhang L.X., Hou H.L., Zhang L., Zhao F.Y., et al. CarcinomaIsocorydine targets the drug-resistant cellular side population through PDCD4-related apoptosis in hepatocellular carcinoma. Mol Med. 2012;18(7):1136–1146. doi: 10.2119/molmed.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L.J., Tian H., Li M., Ge C., Zhao F.Y., Zhang L.X., et al. Derivate isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/M cell cycle arrest and apoptosis. Tumour Biol. 2016;37(5):5951–5961. doi: 10.1007/s13277-015-4362-6. [DOI] [PubMed] [Google Scholar]
- 101.Menezes L.R.A., Costa C.O.D., Rodrigues A., Santo F.R.D., Nepel A., Dutra L.M., et al. Cytotoxic alkaloids from the stem of xylopia laevigata. Molecules. 2016;21(7) doi: 10.3390/molecules21070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh M., Venugopal C., Tokar T., McFarlane N., Subapanditha M.K., Qazi M., et al. Therapeutic Targeting of the Premetastatic Stage in Human Lung-to-Brain Metastasis. Cancer Res. 2018;78(17):5124–5134. doi: 10.1158/0008-5472.CAN-18-1022. [DOI] [PubMed] [Google Scholar]
- 103.Qin Q., Qin J., Chen M., Li Y., Meng T., Zhou J., et al. Chiral platinum (II)-4-(2,3-dihydroxypropyl)-formamide oxoaporphine (FOA) complexes promote tumor cells apoptosis by directly targeting G-quadruplex DNA in vitro and in vivo. Oncotarget. 2017;8(37):61982–61997. doi: 10.18632/oncotarget.18778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Z.F., Qin Q.P., Qin J.L., Liu Y.C., Huang K.B., Li Y.L., et al. Stabilization of G-quadruplex DNA, inhibition of telomerase activity, and tumor cell apoptosis by organoplatinum(II) complexes with oxoisoaporphine. J Med Chem. 2015;58(5):2159–2179. doi: 10.1021/jm5012484. [DOI] [PubMed] [Google Scholar]
- 105.Qin J., Shen W., Chen Z., Zhao L., Qin Q.-P., Yu Y., et al. Oxoaporphine metal complexes (Co-II, Ni-II, Zn-II) with high antitumor activity by inducing mitochondria-mediated apoptosis and S-phase arrest in HepG2. Sci Rep. 2017:7. doi: 10.1038/srep46056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Q.B., Ye R.F., Ye L.Y., Zhang Q.Y., Dai N.G. Isocorydine decrease gemcitabine-resistance by inhibiting epithelial-mesenchymal transition via STAT3 in pancreatic cancer cells. Am J Transl Res. 2020;12(7):3702–3714. [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou L., Wang Q.Y., Zhang H., Li Y.J., Xie S.Y., Xu M.L. YAP inhibition by nuciferine via AMPK-mediated downregulation of HMGCR sensitizes pancreatic cancer cells to gemcitabine. Biomolecules. 2019;9(10) doi: 10.3390/biom9100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu R.M., Xu P., Chen Q., Feng S.L., Xie Y. A multiple-targets alkaloid nuciferine overcomes paclitaxel-induced drug resistance in vitro and in vivo. Phytomedicine. 2020:79. doi: 10.1016/j.phymed.2020.153342. [DOI] [PubMed] [Google Scholar]
- 109.Bashir DJ, Manzoor S, Sarfaraj M, Afzal SM, Bashir M, Nidhi, et al. Magnoflorine-Loaded Chitosan Collagen Nanocapsules Ameliorate Cognitive Deficit in Scopolamine-Induced Alzheimer’s Disease-like Conditions in a Rat Model by Downregulating IL-1β, IL-6, TNF-α, and Oxidative Stress and Upregulating Brain-Derived Neurotrophic Factor and DCX Expressions. ACS Omega. 2023;8(2):2227-36. doi: 10.1021/acsomega.2c06467. [DOI] [PMC free article] [PubMed]
- 110.Sun X., Zhang X., Zhai H., Zhang D., Ma S. Magnoflorine inhibits human gastric cancer progression by inducing autophagy, apoptosis and cell cycle arrest by JNK activation regulated by ROS. Biomed Pharmacother. 2020:125. doi: 10.1016/j.biopha.2019.109118. [DOI] [PubMed] [Google Scholar]
- 111.Oh M., Lee J., Shin D., Park J., Oian T., Kim H., et al. The in vitro and in vivo anti-tumor effect of KO-202125, a sauristolactam derivative, as a novel epidermal growth factor receptor inhibitor in human breast cancer. Cancer Sci. 2011;102(3):597–604. doi: 10.1111/j.1349-7006.2010.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X., Cheang W.S., Yang H., Xiao L., Lai B., Zhang M., et al. Nuciferine relaxes rat mesenteric arteries through endothelium-dependent and -independent mechanisms. Br J Pharmacol. 2015;172(23):5609–5618. doi: 10.1111/bph.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L., Li P.F., Zhou Y., Gu R.J., Lu G., Zhang C.B. Magnoflorine Ameliorates Collagen-Induced Arthritis by Suppressing the Inflammation Response via the NF-kappa B/MAPK Signaling Pathways. J Inflamm Res. 2023;16:2271–2296. doi: 10.2147/JIR.S406298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tomsik P., Micuda S., Muthna D., Cermakova E., Havelek R., Rudolf E., et al. Boldine inhibits mouse mammary carcinoma in vivo and human MCF-7 breast cancer cells in vitro. Planta Med. 2016;82(16):1416–1424. doi: 10.1055/s-0042-113611. [DOI] [PubMed] [Google Scholar]
- 115.Qu L., Liu Q., Zhang Q., Tuo X., Fan D., Deng J., et al. Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem Toxicol. 2019;125:85–94. doi: 10.1016/j.fct.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 116.Zhang X., Zhou Y., Cheong M.S., Khan H., Ruan C.-C., Fu M., et al. Citri Reticulatae Pericarpium extract and flavonoids reduce inflammation in RAW 264.7 macrophages by inactivation of MAPK and NF-κB pathways. Food Frontiers. 2022;3(4):785–795. doi: 10.1002/fft2.169. [DOI] [Google Scholar]
- 117.Xu J., Ying A., Shi T. Nuciferine inhibits skin cutaneous melanoma cell growth by suppressing TLR4/NF-kappa B signaling. Anticancer Agents Med Chem. 2020;20(17):2099–2105. doi: 10.2174/1871520620666200811114607. [DOI] [PubMed] [Google Scholar]
- 118.Okon E., Kukula-Koch W., Jarzab A., Halasa M., Stepulak A., Wawruszak A. Advances in chemistry and bioactivity of magnoflorine and magnoflorine-containing extracts. Int J Mol Sci. 2020;21(4) doi: 10.3390/ijms21041330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun H., Hou H., Lu P., Zhang L., Zhao F., Ge C., et al. Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/M cell cycle arrest and apoptosis. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paydar M., Kamalidehghan B., Wong Y.L., Wong W.F., Looi C.Y., Mustafa M.R. Evaluation of cytotoxic and chemotherapeutic properties of boldine in breast cancer using in vitro and in vivo models. Drug Des Devel Ther. 2014;8:719–733. doi: 10.2147/DDDT.S58178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu P., Chiu C., Chen C., Wang H.D. 7-Hydroxydehydronuciferine induces human melanoma death via triggering autophagy and apoptosis. Exp Dermatol. 2015;24(12):930–935. doi: 10.1111/exd.12805. [DOI] [PubMed] [Google Scholar]
- 122.Santos L.S., Silva V.R., Alencar Menezes L.R., Pereira Soares M.B., Costa E.V., Bezerra D.P. Xylopine induces oxidative stress and causes G(2)/M phase arrest, triggering caspase-mediated apoptosis by p53-independent pathway in HCT116 cells. Oxid Med Cell Longevity. 2017; 2017. doi: 10.1155/2017/7126872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Konkimalla V.B., Efferth T. Inhibition of epidermal growth factor receptor over-expressing cancer cells by the aphorphine-type isoquinoline alkaloid, dicentrine. Biochem Pharmacol. 2010;79(8):1092–1099. doi: 10.1016/j.bcp.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 124.Noureini S.K., Kheirabadi M., Masoumi F., Khosrogerdi F., Zarei Y., Suarez-Rozas C., et al. Telomerase inhibition by a new synthetic derivative of the aporphine alkaloid boldine. Int J Mol Sci. 2018;19(4) doi: 10.3390/ijms19041239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Noureini S.K., Tanavar F. Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations. Chem Biol Interact. 2015;231:27–34. doi: 10.1016/j.cbi.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 126.Yu L., Han S., Lang L., Song H., Zhang C., Dong L., et al. Oxocrebanine: A novel dual topoisomerase inhibitor, suppressed the proliferation of breast cancer cells MCF-7 by inducing DNA damage and mitotic arrest. Phytomedicine. 2021:84. doi: 10.1016/j.phymed.2021.153504. [DOI] [PubMed] [Google Scholar]
- 127.Tran T.H.T., Vu L.D.B., Nguyen H.Q., Pham H.B., Do X.P.T., Than U.T.T., et al. Dual roles of oxostephanine as an Aurora kinase inhibitor and angiogenesis suppressor. Int J Mol Med. 2022;50(5) doi: 10.3892/ijmm.2022.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Branches A.D.S., Costa R.A., Junior E.S.A., Bezzera D.P., Soares M.B.P., Costa E.V., et al. Theoretical and experimental study by DFT, molecular docking calculations and cytotoxicity assay of 7,7-dimethylaporphine alkaloids type isolated from Guatteria friesiana (Annonaceae) J Mol Struct. 2019;1177:347–362. doi: 10.1016/j.molstruc.2018.09.060. [DOI] [Google Scholar]
- 129.Costa R.A., Barros G.A., da Silva J.N., Oliveira K.M., Bezerra D.P., Soares M.B.P., et al. Experimental and theoretical study on spectral features, reactivity, solvation, topoisomerase I inhibition and in vitro cytotoxicity in human HepG2 cells of guadiscine and guadiscidine aporphine alkaloids. J Mol Struct. 2021;1229 doi: 10.1016/j.molstruc.2020.129844. [DOI] [Google Scholar]
- 130.Matson V., Chervin C.S., Gajewski T.F. Cancer and the Microbiome—Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology. 2021;160(2):600–613. doi: 10.1053/j.gastro.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y., Tong Q., Ma S.-R., Zhao Z.-X., Pan L.-B., Cong L., et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduction Targeted Ther. 2021;6(1):77. doi: 10.1038/s41392-020-00456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tiwari S., Awasthi M., Singh S., Pandey V.P., Dwivedi U.N. Modulation of interaction of mutant TP53 and wild type BRCA1 by alkaloids: a computational approach towards targeting protein-protein interaction as a futuristic therapeutic intervention strategy for breast cancer impediment. J Biomol Struct Dyn. 2018;36(13):3376–3387. doi: 10.1080/07391102.2017.1388286. [DOI] [PubMed] [Google Scholar]
- 133.Mollazadeh S., Mackiewicz M., Yazdimamaghani M. Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective. Mater Sci Eng C Mater Biol Appl. 2021;118 doi: 10.1016/j.msec.2020.111536. [DOI] [PubMed] [Google Scholar]
- 134.Jin Q., Yang D., Dai Z., Khan A., Wang B., Wei X., et al. Antitumor aporphine alkaloids from Thalictrum wangii. Fitoterapia. 2018;128:204–212. doi: 10.1016/j.fitote.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 135.Sesang W., Punyanitya S., Pitchuanchom S., Udomputtimekakul P., Nuntasaen N., Banjerdpongchai R., et al. Cytotoxic aporphine alkaloids from leaves and twigs of pseuduvaria trimera (craib) Molecules. 2014;19(7):8762–8772. doi: 10.3390/molecules19078762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lekphrom R., Kanokmedhakul S., Kanokmedhakul K. Bioactive styryllactones and alkaloid from flowers of Goniothalamus laoticus. J Ethnopharmacol. 2009;125(1):47–50. doi: 10.1016/j.jep.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 137.Garcez F.R., da Silva A.F.G., Garcez W.S., Linck G., Matos M.D.C., Santos E.C.S., et al. Cytotoxic aporphine alkaloids from ocotea acutifolia. Planta Med. 2011;77(4):383–387. doi: 10.1055/s-0030-1250401. [DOI] [PubMed] [Google Scholar]
- 138.Zahari A., Cheah F.K., Mohamad J., Sulaiman S.N., Litaudon M., Leong K.H., et al. Antiplasmodial and antioxidant isoquinoline alkaloids from dehaasia longipedicellata. Planta Med. 2014;80(7):599–603. doi: 10.1055/s-0034-1368349. [DOI] [PubMed] [Google Scholar]
- 139.Makarasen A., Sirithana W., Mogkhuntod S., Khunnawutmanotham N., Chimnoi N., Techasakul S. Cytotoxic and antimicrobial activities of aporphine alkaloids isolated from stephania venosa (blume) spreng. Planta Med. 2011;77(13):1519–1524. doi: 10.1055/s-0030-1270743. [DOI] [PubMed] [Google Scholar]
- 140.Le P.M., Srivastava V., Nguyen T.T., Pradines B., Madamet M., Mosnier J., et al. Stephanine from stephania venosa (blume) spreng showed effective antiplasmodial and anticancer activities, the latter by inducing apoptosis through the reverse of mitotic exit. Phytother Res. 2017;31(9):1357–1368. doi: 10.1002/ptr.5861. [DOI] [PubMed] [Google Scholar]
- 141.Thijssen E., Den Heijer J.M., Puibert D., Van Brummelen E.M.J., Naranda T., Jan G.G. Safety and pharmacokinetics of multiple dosing with inhalable apomorphine (AZ-009), and its efficacy in a randomized crossover study in Parkinson's disease patients. Parkinsonism Relat Disord. 2022;97:84–90. doi: 10.1016/j.parkreldis.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 142.Zhou J., Sun J.B., Zheng P., Liu J., Cheng Z.H., Zeng P., et al. Orthogonal array design for optimization of hollow-fiber-based liquid-phase microextraction combined with high-performance liquid chromatography for study of the pharmacokinetics of magnoflorine in rat plasma. Anal Bioanal Chem. 2012;403(7):1951–1960. doi: 10.1007/s00216-012-6013-8. [DOI] [PubMed] [Google Scholar]
- 143.Lu J.Z., Hong D.D., Ye D., Mu S., Shi R., Song Y., et al. Tissue distribution and integrated pharmacokinetic properties of major effective constituents of oral Gegen-Qinlian decoction in mice. Front Pharmacol. 2022:13. doi: 10.3389/fphar.2022.996143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu Y.L., He Y.Q., Wang M.A., Zhang L., Yang L., Wang Z.T., et al. Characterization of nuciferine metabolism by P450 enzymes and uridine diphosphate glucuronosyltransferases in liver microsomes from humans and animals. Acta Pharmacol Sin. 2010;31(12):1635–1642. doi: 10.1038/aps.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qin Q., Qin J., Meng T., Lin W., Zhang C., Wei Z., et al. High in vivo antitumor activity of cobalt oxoisoaporphine complexes by targeting G-quadruplex DNA, telomerase and disrupting mitochondrial functions. Eur J Med Chem. 2016;124:380–392. doi: 10.1016/j.ejmech.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 146.Lin H., Huang H., Liao J., Shen C., Huang R. Dicentrine analogue-induced G2/M arrest and apoptosis through inhibition of topoisomerase II activity in human cancer cells. Planta Med. 2015;81(10):830–837. doi: 10.1055/s-0035-1546128. [DOI] [PubMed] [Google Scholar]
- 147.Yodkeeree S., Pompimon W., Limtrakul P. Crebanine, an aporphine alkaloid, sensitizes TNF-alpha-induced apoptosis and suppressed invasion of human lung adenocarcinoma cells A549 by blocking NF-kappa B-regulated gene products. Tumour Biol. 2014;35(9):8615–8624. doi: 10.1007/s13277-014-1998-6. [DOI] [PubMed] [Google Scholar]