Abstract
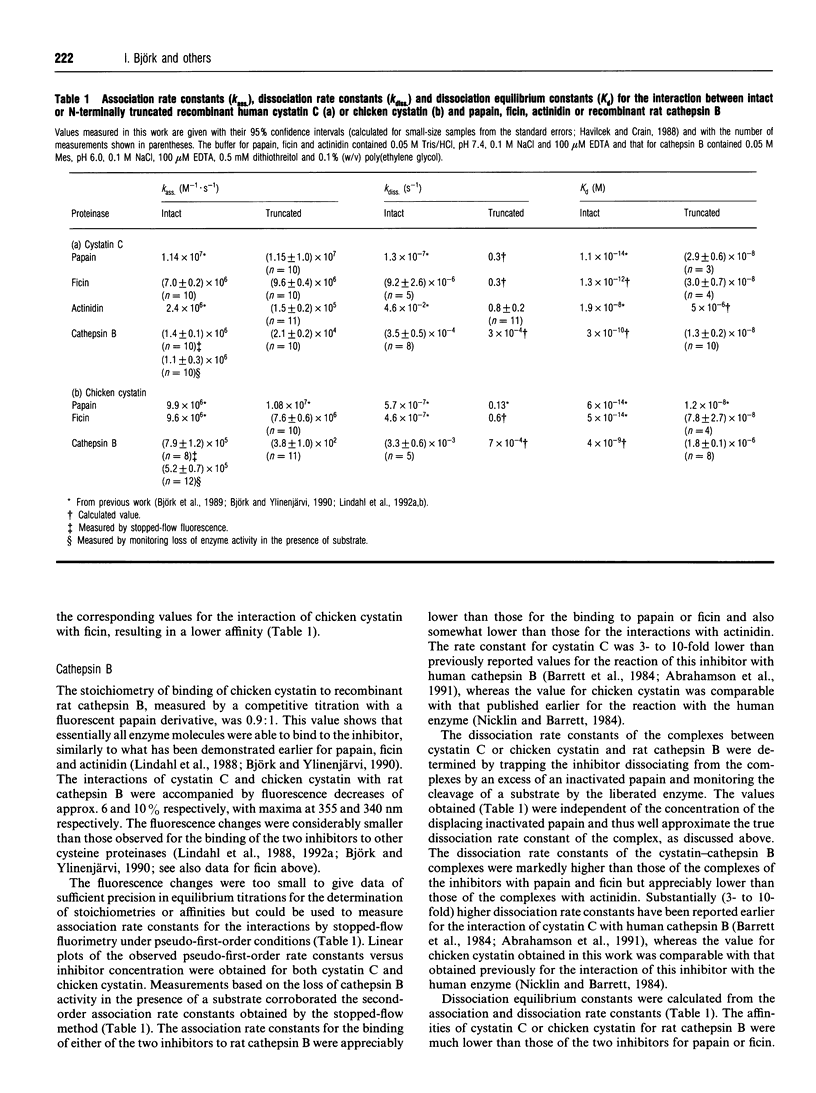
The importance of the N-terminal region of human cystatin C or chicken cystatin for the kinetics of interactions of the inhibitors with four cysteine proteinases was characterized. The association rate constants for the binding of recombinant human cystatin C to papain, ficin, actinidin and recombinant rat cathepsin B were 1.1 x 10(7), 7.0 x 10(6), 2.4 x 10(6) and 1.4 x 10(6) M-1.s-1, whereas the corresponding dissociation rate constants were 1.3 x 10(-7), 9.2 x 10(-6), 4.6 x 10(-2) and 3.5 x 10(-4) s-1. N-Terminal truncation of the first ten residues of the inhibitor negligibly affected the association rate constant with papain or ficin, but increased the dissociation rate constant approx. 3 x 10(4)- to 2 x 10(6)-fold. In contrast, such truncation decreased the association rate constant with cathepsin B approx. 60-fold, while minimally affecting the dissociation rate constant. With actinidin, the truncated cystatin C had both an approx. 15-fold lower association rate constant and an approx. 15-fold higher dissociation rate constant than the intact inhibitor. Similar results were obtained for intact and N-terminally truncated chicken cystatin. The decreased affinity of human cystatin C or chicken cystatin for cysteine proteinases after removal of the N-terminal region is thus due to either a decreased association rate constant or an increased dissociation rate constant, or both, depending on the enzyme. This behaviour indicates that the contribution of the N-terminal segment of the two inhibitors to the interaction mechanism varies with the target proteinase as a result of structural differences in the active-site region of the enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988 Aug 15;236(1):14–18. doi: 10.1016/0014-5793(88)80276-x. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Mason R. W., Hansson H., Buttle D. J., Grubb A., Ohlsson K. Human cystatin C. role of the N-terminal segment in the inhibition of human cysteine proteinases and in its inactivation by leucocyte elastase. Biochem J. 1991 Feb 1;273(Pt 3):621–626. doi: 10.1042/bj2730621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald E. A., Genenger G., Assfalg-Machleidt I., Machleidt W., Engh R. A., Fritz H. Recombinant chicken egg white cystatin variants of the QLVSG region. Eur J Biochem. 1992 Nov 1;209(3):837–845. doi: 10.1111/j.1432-1033.1992.tb17355.x. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Davies M. E., Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984 Apr 30;120(2):631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Björk I., Alriksson E., Ylinenjärvi K. Kinetics of binding of chicken cystatin to papain. Biochemistry. 1989 Feb 21;28(4):1568–1573. doi: 10.1021/bi00430a022. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction between chicken cystatin and the cysteine proteinases actinidin, chymopapain A, and ficin. Biochemistry. 1990 Feb 20;29(7):1770–1776. doi: 10.1021/bi00459a016. [DOI] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Laber B., Stubbs M., Huber R., Turk V. Mechanism of interaction of cysteine proteinases and their protein inhibitors as compared to the serine proteinase-inhibitor interaction. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):111–118. [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors-I. Kinetic behavior. Biochem Pharmacol. 1975 Dec 1;24(23):2177–2185. doi: 10.1016/0006-2952(75)90050-7. [DOI] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Wolthers B. G. The structure of papain. Adv Protein Chem. 1971;25:79–115. doi: 10.1016/s0065-3233(08)60279-x. [DOI] [PubMed] [Google Scholar]
- Hasnain S., Hirama T., Tam A., Mort J. S. Characterization of recombinant rat cathepsin B and nonglycosylated mutants expressed in yeast. New insights into the pH dependence of cathepsin B-catalyzed hydrolyses. J Biol Chem. 1992 Mar 5;267(7):4713–4721. [PubMed] [Google Scholar]
- Lindahl P., Abrahamson M., Björk I. Interaction of recombinant human cystatin C with the cysteine proteinases papain and actinidin. Biochem J. 1992 Jan 1;281(Pt 1):49–55. doi: 10.1042/bj2810049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Alriksson E., Jörnvall H., Björk I. Interaction of the cysteine proteinase inhibitor chicken cystatin with papain. Biochemistry. 1988 Jul 12;27(14):5074–5082. doi: 10.1021/bi00414a019. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Nycander M., Ylinenjärvi K., Pol E., Björk I. Characterization by rapid-kinetic and equilibrium methods of the interaction between N-terminally truncated forms of chicken cystatin and the cysteine proteinases papain and actinidin. Biochem J. 1992 Aug 15;286(Pt 1):165–171. doi: 10.1042/bj2860165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Raub-Segall E., Olson S. T., Björk I. Papain labelled with fluorescent thiol-specific reagents as a probe for characterization of interactions between cysteine proteinases and their protein inhibitors by competitive titrations. Biochem J. 1991 Jun 1;276(Pt 2):387–394. doi: 10.1042/bj2760387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Musil D., Zucic D., Turk D., Engh R. A., Mayr I., Huber R., Popovic T., Turk V., Towatari T., Katunuma N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO J. 1991 Sep;10(9):2321–2330. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Barrett A. J. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984 Oct 1;223(1):245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nycander M., Björk I. Evidence by chemical modification that tryptophan-104 of the cysteine-proteinase inhibitor chicken cystatin is located in or near the proteinase-binding site. Biochem J. 1990 Oct 1;271(1):281–284. doi: 10.1042/bj2710281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popović T., Brzin J., Ritonja A., Turk V. Different forms of human cystatin C. Biol Chem Hoppe Seyler. 1990 Jul;371(7):575–580. doi: 10.1515/bchm3.1990.371.2.575. [DOI] [PubMed] [Google Scholar]
- Romanos M. A., Beesley K. M., Clare J. J. Direct selection of stabilised yeast URA3 transformants with 5-fluorouracil. Nucleic Acids Res. 1991 Jan 11;19(1):187–187. doi: 10.1093/nar/19.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. D., Feng R., Konishi Y., Mort J. S. Demonstration by electrospray mass spectrometry that the peptidyldipeptidase activity of cathepsin B is capable of rat cathepsin B C-terminal processing. Biochem J. 1993 Sep 15;294(Pt 3):923–927. doi: 10.1042/bj2940923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. D., Mason P., Mach L., Mort J. S. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem. 1992 Aug 5;267(22):15993–15999. [PubMed] [Google Scholar]
- San Segundo B., Chan S. J., Steiner D. F. Identification of cDNA clones encoding a precursor of rat liver cathepsin B. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2320–2324. doi: 10.1073/pnas.82.8.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Maggio E. T., Kenyon G. L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry. 1975 Feb 25;14(4):766–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]


