Abstract
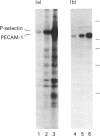
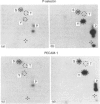
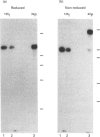
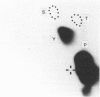
P-selectin is a 140 kDa membrane glycoprotein found in secretory granules of platelets and endothelial cells where it is rapidly translocated to the plasma membrane upon cell activation. It then functions as a receptor for various types of leucocytes. Metabolic labelling of resting platelets with 32Pi showed that P-selectin is primarily phosphorylated on serine residues, although some tyrosine phosphorylation was observed as well. However, tyrosine phosphorylation of P-selectin was greatly stimulated by treatment with the permeating phosphatase inhibitor, pervanadate. When P-selectin immunoprecipitates were incubated with [gamma-32P]ATP (in vitro kinase assay), a fraction of P-selectin was phosphorylated on its tyrosine residues by a co-precipitated kinase. P-selectin phosphorylated in vitro co-migrated with 140 kDa surface-labelled 125I-P-selectin during SDS/PAGE under reducing conditions. Under non-reducing conditions, however, phosphorylated P-selectin was disulphide-linked to unknown protein(s) in a 205 kDa complex. In vitro kinase assays of the most abundant platelet tyrosine kinase, pp60c-src, demonstrated the presence of similar 140 and 205 kDa phosphorylated proteins in SDS/PAGE under reducing and non-reducing conditions respectively. Extraction and reprecipitation studies with proteins phosphorylated in vitro indicated that P-selectin and pp60c-src form a 205 kDa 1:1 disulphide-linked complex. In the complex, pp60c-src autophosphorylation is inhibited and P-selectin is phosphorylated on tyrosine residues. As protein disulphides in the cytoplasm of intact cells are extremely rare, our results suggest that P-selectin and pp60c-src, which co-localize in platelet dense granules, may be non-covalently associated and spontaneously form disulphide bridges during lysis. In addition, the observed tyrosine phosphorylation of P-selectin in intact platelets suggests that its function might be regulated by phosphorylation by pp60c-src.
Full text
PDF


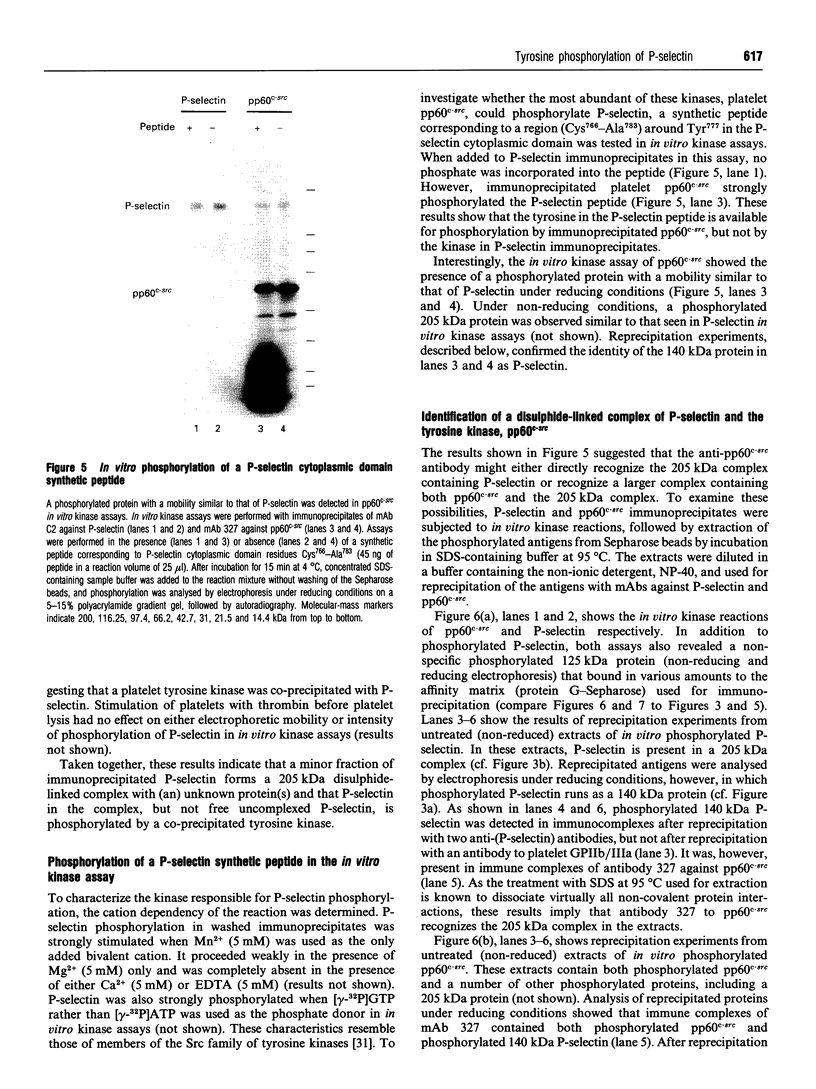
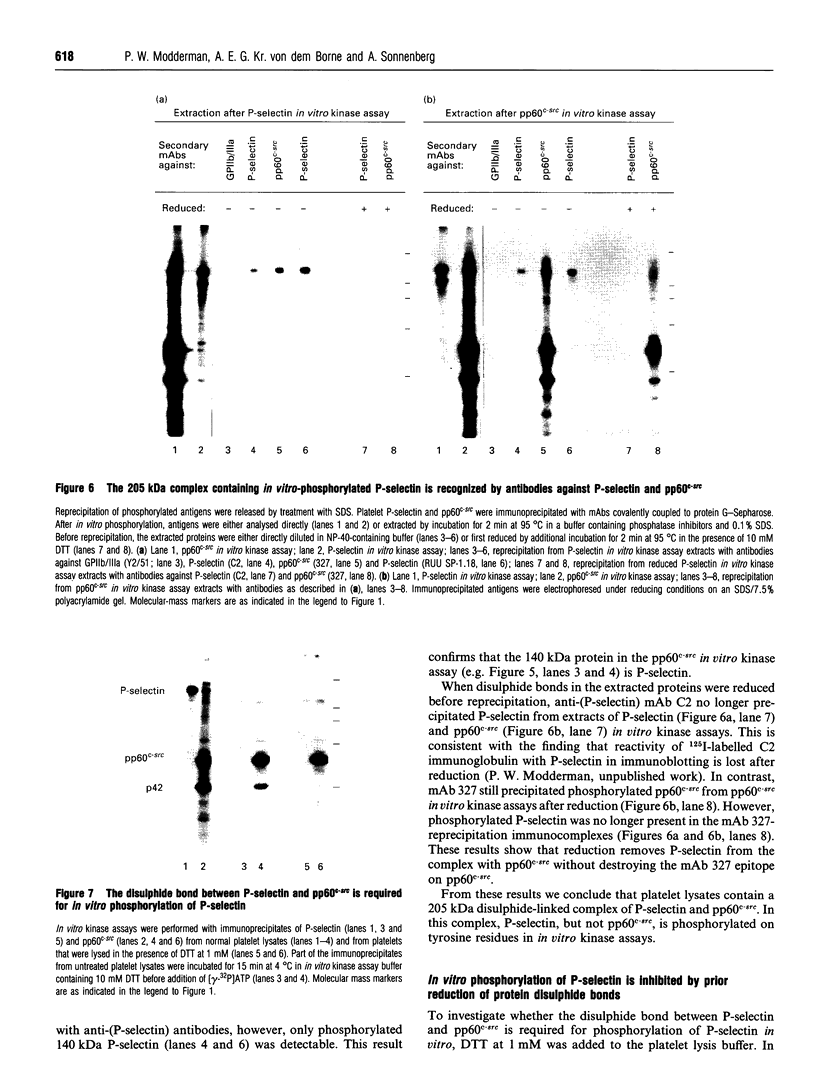



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R. A., Walker T. R., Watson S. P. Activation of human platelets by peroxovanadate is associated with tyrosine phosphorylation of phospholipase C gamma and formation of inositol phosphates. Biochem J. 1993 Mar 1;290(Pt 2):471–475. doi: 10.1042/bj2900471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Israel M. A. In vitro association and phosphorylation of polyoma virus middle T antigen by cellular tyrosyl kinase activity. J Biol Chem. 1984 Oct 10;259(19):11686–11694. [PubMed] [Google Scholar]
- Bolen J. B., Rowley R. B., Spana C., Tsygankov A. Y. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992 Dec;6(15):3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Nishio H., Hatase O., Ralph S., Wang J. H. A synthetic peptide derived from p34cdc2 is a specific and efficient substrate of src-family tyrosine kinases. J Biol Chem. 1992 May 5;267(13):9248–9256. [PubMed] [Google Scholar]
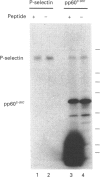
- Crovello C. S., Furie B. C., Furie B. Rapid phosphorylation and selective dephosphorylation of P-selectin accompanies platelet activation. J Biol Chem. 1993 Jul 15;268(20):14590–14593. [PubMed] [Google Scholar]
- Disdier M., Morrissey J. H., Fugate R. D., Bainton D. F., McEver R. P. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol Biol Cell. 1992 Mar;3(3):309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe D. V., Watson S. R., Presta L. G., Wolitzky B. A., Foxall C., Brandley B. K., Lasky L. A. P- and E-selectin use common sites for carbohydrate ligand recognition and cell adhesion. J Cell Biol. 1993 Mar;120(5):1227–1235. doi: 10.1083/jcb.120.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantus I. G., Kadota S., Deragon G., Foster B., Posner B. I. Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry. 1989 Oct 31;28(22):8864–8871. doi: 10.1021/bi00448a027. [DOI] [PubMed] [Google Scholar]
- Feder D., Bishop J. M. Purification and enzymatic characterization of pp60c-src from human platelets. J Biol Chem. 1990 May 15;265(14):8205–8211. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Noble J. A., Martin G. S., Jacques Y. V., Bainton D. F. Intracellular localization of pp60c-src in human platelets. Oncogene. 1990 Jul;5(7):1033–1036. [PubMed] [Google Scholar]
- Fujimoto T., Stroud E., Whatley R. E., Prescott S. M., Muszbek L., Laposata M., McEver R. P. P-selectin is acylated with palmitic acid and stearic acid at cysteine 766 through a thioester linkage. J Biol Chem. 1993 May 25;268(15):11394–11400. [PubMed] [Google Scholar]
- Fukazawa H., Mizuno S., Uehara Y. Effects of herbimycin A and various SH-reagents on p60v-src kinase activity in vitro. Biochem Biophys Res Commun. 1990 Nov 30;173(1):276–282. doi: 10.1016/s0006-291x(05)81053-8. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Geng J. G., Heavner G. A., McEver R. P. Lectin domain peptides from selectins interact with both cell surface ligands and Ca2+ ions. J Biol Chem. 1992 Oct 5;267(28):19846–19853. [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Grandori C., Hanafusa H. p60c-src is complexed with a cellular protein in subcellular compartments involved in exocytosis. J Cell Biol. 1988 Dec;107(6 Pt 1):2125–2135. doi: 10.1083/jcb.107.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger S. A., McEver R. P. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990 Feb 1;75(3):550–554. [PubMed] [Google Scholar]
- Hollenbaugh D., Bajorath J., Stenkamp R., Aruffo A. Interaction of P-selectin (CD62) and its cellular ligand: analysis of critical residues. Biochemistry. 1993 Mar 30;32(12):2960–2966. doi: 10.1021/bi00063a006. [DOI] [PubMed] [Google Scholar]
- Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. M., Indik Z., Brass L. F., Hoxie J. A., Schreiber A. D., Brugge J. S. Activation of Fc gamma RII induces tyrosine phosphorylation of multiple proteins including Fc gamma RII. J Biol Chem. 1992 Mar 15;267(8):5467–5473. [PubMed] [Google Scholar]
- Inazu T., Taniguchi T., Yanagi S., Yamamura H. Protein-tyrosine phosphorylation and aggregation of intact human platelets by vanadate with H2O2. Biochem Biophys Res Commun. 1990 Jul 16;170(1):259–263. doi: 10.1016/0006-291x(90)91268-w. [DOI] [PubMed] [Google Scholar]
- Israels S. J., Gerrard J. M., Jacques Y. V., McNicol A., Cham B., Nishibori M., Bainton D. F. Platelet dense granule membranes contain both granulophysin and P-selectin (GMP-140). Blood. 1992 Jul 1;80(1):143–152. [PubMed] [Google Scholar]
- Issekutz A. C., Ripley M., Jackson J. R. Role of neutrophils in the deposition of platelets during acute inflammation. Lab Invest. 1983 Dec;49(6):716–724. [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Ley K., Munro J. M., Tedder T. F. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J Exp Med. 1993 Mar 1;177(3):833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen E., Celi A., Gilbert G. E., Furie B. C., Erban J. K., Bonfanti R., Wagner D. D., Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989 Oct 20;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lerea K. M., Tonks N. K., Krebs E. G., Fischer E. H., Glomset J. A. Vanadate and molybdate increase tyrosine phosphorylation in a 50-kilodalton protein and stimulate secretion in electropermeabilized platelets. Biochemistry. 1989 Nov 28;28(24):9286–9292. doi: 10.1021/bi00450a008. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Leukocyte interactions mediated by selectins. Thromb Haemost. 1991 Jul 12;66(1):80–87. [PubMed] [Google Scholar]
- McEver R. P. Leukocyte-endothelial cell interactions. Curr Opin Cell Biol. 1992 Oct;4(5):840–849. doi: 10.1016/0955-0674(92)90109-p. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- Modderman P. W., Admiraal L. G., Sonnenberg A., von dem Borne A. E. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem. 1992 Jan 5;267(1):364–369. [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Hillery C. A., Albrecht R., Parise L. V., Berndt M. C., Mazurov A. V., Dunlop L. C., Zhang J., Rittenhouse S. E. Activation-dependent changes in human platelet PECAM-1: phosphorylation, cytoskeletal association, and surface membrane redistribution. J Cell Biol. 1992 Oct;119(1):239–246. doi: 10.1083/jcb.119.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard K. E., Moore K. L., Diaz S., Stults N. L., Ushiyama S., McEver R. P., Cummings R. D., Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993 Jun 15;268(17):12764–12774. [PubMed] [Google Scholar]
- Palabrica T., Lobb R., Furie B. C., Aronovitz M., Benjamin C., Hsu Y. M., Sajer S. A., Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992 Oct 29;359(6398):848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- Pumiglia K. M., Lau L. F., Huang C. K., Burroughs S., Feinstein M. B. Activation of signal transduction in platelets by the tyrosine phosphatase inhibitor pervanadate (vanadyl hydroperoxide). Biochem J. 1992 Sep 1;286(Pt 2):441–449. doi: 10.1042/bj2860441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F., Lebret M., Danielian S., Fagard R., Levy-Toledano S., Fischer S. High pp60c-src level in human platelet dense bodies. Blood. 1989 May 1;73(6):1545–1551. [PubMed] [Google Scholar]
- Sanders W. E., Wilson R. W., Ballantyne C. M., Beaudet A. L. Molecular cloning and analysis of in vivo expression of murine P-selectin. Blood. 1992 Aug 1;80(3):795–800. [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Strubel N. A., Nguyen M., Kansas G. S., Tedder T. F., Bischoff J. Isolation and characterization of a bovine cDNA encoding a functional homolog of human P-selectin. Biochem Biophys Res Commun. 1993 Apr 30;192(2):338–344. doi: 10.1006/bbrc.1993.1420. [DOI] [PubMed] [Google Scholar]
- Weller A., Isenmann S., Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J Biol Chem. 1992 Jul 25;267(21):15176–15183. [PubMed] [Google Scholar]
- Zehnder J. L., Hirai K., Shatsky M., McGregor J. L., Levitt L. J., Leung L. L. The cell adhesion molecule CD31 is phosphorylated after cell activation. Down-regulation of CD31 in activated T lymphocytes. J Biol Chem. 1992 Mar 15;267(8):5243–5249. [PubMed] [Google Scholar]
- de Bruijne-Admiraal L. G., Modderman P. W., Von dem Borne A. E., Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood. 1992 Jul 1;80(1):134–142. [PubMed] [Google Scholar]
- van Mourik J. A., Leeksma O. C., Reinders J. H., de Groot P. G., Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J Biol Chem. 1985 Sep 15;260(20):11300–11306. [PubMed] [Google Scholar]