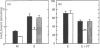Abstract
The mechanism by which Ca2+ inhibits InsP3-induced Ca2+ release from sarcoplasmic reticulum of vascular smooth muscle was investigated. InsP3 binding to sarcoplasmic-reticulum vesicles from dog aortic smooth muscle was inhibited by 51 +/- 6% by 2 microM Ca2+ in the presence of 10 nM [3H]InsP3. Scatchard analysis indicated the presence of two InsP3-binding sites in the absence of Ca2+ (Kd = 2.5 +/- 0.9 and 49 +/- 8 nM InsP3), though the low-affinity site was more prevalent (representing 92 +/- 3% of the total number of binding sites). Ca2+ (2 microM) did not alter InsP3 binding to the high-affinity site (P > 0.05), but increased the Kd of the low-affinity site 3-fold (Kd = 155 +/- 4 nM InsP3; P < 0.001). The possibility that the apparent decrease in InsP3 affinity was caused by Ca(2+)-dependent activation of an endogenous phospholipase C could be excluded, because the Ca(2+)-dependent inhibition of InsP3 binding was completely reversible and insensitive to an inhibitor of phospholipase C. Moreover, Ca2+ did not inhibit InsP3 binding to InsP3 receptor partially purified by heparin-Sepharose chromatography, though another fraction (devoid of InsP3 receptor) restored Ca(2+)-sensitivity of the partially purified InsP3 receptor. Thus Ca2+ binding to a Ca(2+)-sensitizing factor associated with the InsP3 receptor decreases the affinity of the receptor complex for InsP3. This Ca(2+)-sensitizing factor may provide a negative-feedback mechanism for regulating the rise in cytosolic Ca2+ concentration in vascular smooth muscle after hormone activation of the phosphoinositide cascade.
Full text
PDF
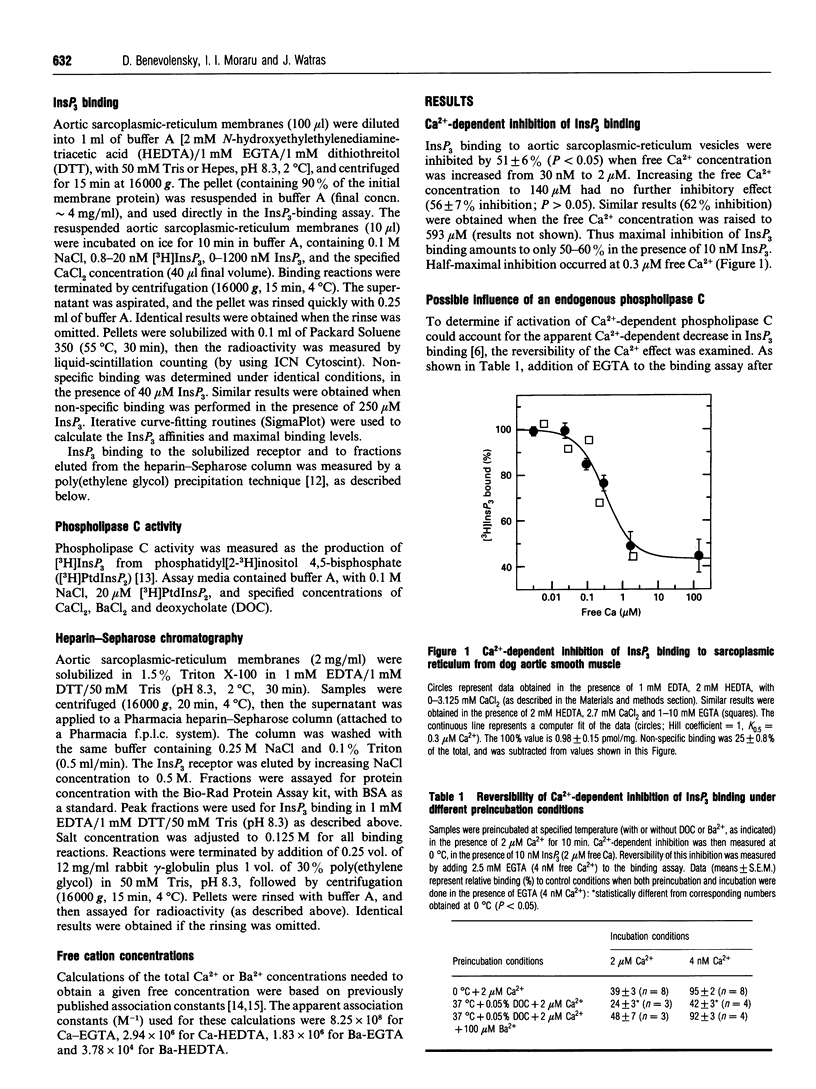
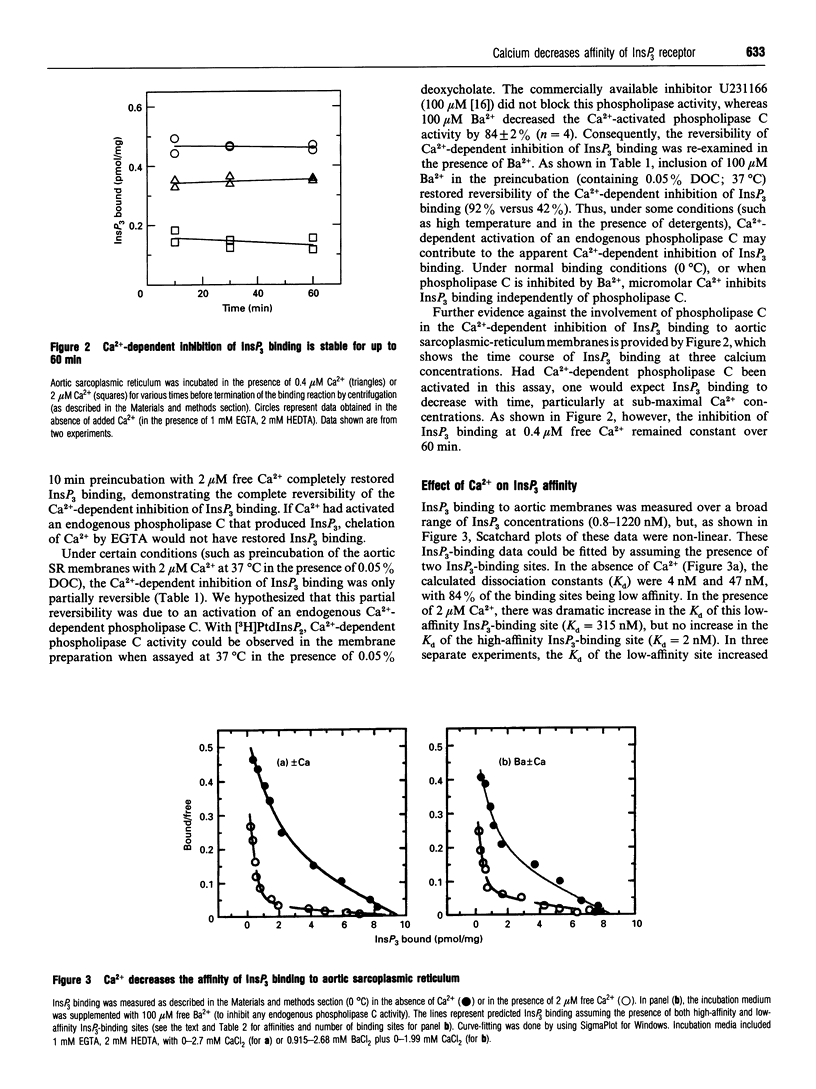
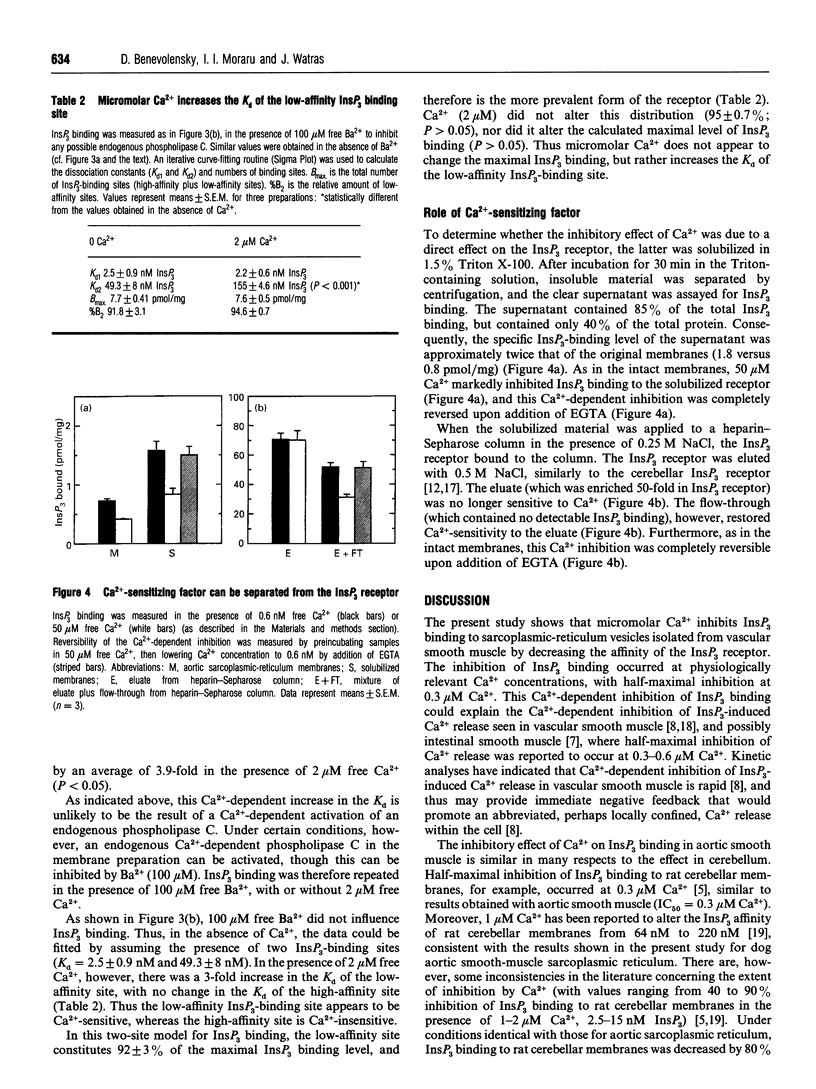


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Banno Y., Nakashima S., Nozawa Y. Partial purification of phosphoinositide phospholipase C from human platelet cytosol; characterization of its three forms. Biochem Biophys Res Commun. 1986 Apr 29;136(2):713–721. doi: 10.1016/0006-291x(86)90498-5. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bleasdale J. E., Thakur N. R., Gremban R. S., Bundy G. L., Fitzpatrick F. A., Smith R. J., Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990 Nov;255(2):756–768. [PubMed] [Google Scholar]
- Chadwick C. C., Saito A., Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoff S. K., Ferris C. D., Donath C., Fischer G. A., Munemitsu S., Ullrich A., Snyder S. H., Ross C. A. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoff S. K., Supattapone S., Snyder S. H. Characterization of a membrane protein from brain mediating the inhibition of inositol 1,4,5-trisphosphate receptor binding by calcium. Biochem J. 1988 Sep 15;254(3):701–705. doi: 10.1042/bj2540701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Snyder S. H. Inositol phosphate receptors and calcium disposition in the brain. J Neurosci. 1992 May;12(5):1567–1574. doi: 10.1523/JNEUROSCI.12-05-01567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L., Williamson J. R. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J. 1989 Feb 15;258(1):261–265. doi: 10.1042/bj2580261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Johnston P. A., Südhof T. C. Mechanism of Ca2+ inhibition of inositol 1,4,5-trisphosphate (InsP3) binding to the cerebellar InsP3 receptor. J Biol Chem. 1992 Apr 15;267(11):7450–7455. [PubMed] [Google Scholar]
- Mourey R. J., Verma A., Supattapone S., Snyder S. H. Purification and characterization of the inositol 1,4,5- trisphosphate receptor protein from rat vas deferens. Biochem J. 1990 Dec 1;272(2):383–389. doi: 10.1042/bj2720383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Okano H., Furuichi T., Aruga J., Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally specific manner. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri F., Hilly M., Mauger J. P. Calcium mediates the interconversion between two states of the liver inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Oct 15;265(29):17478–17485. [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Sasaguri T., Hashimoto T., Kuriyama H. Roles of Ca2+ on the inositol 1,4,5-trisphosphate-induced release of Ca2+ from saponin-permeabilized single cells of the porcine coronary artery. Comp Biochem Physiol A Comp Physiol. 1985;82(3):645–649. doi: 10.1016/0300-9629(85)90446-3. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Taylor C. W., Richardson A. Structure and function of inositol trisphosphate receptors. Pharmacol Ther. 1991;51(1):97–137. doi: 10.1016/0163-7258(91)90043-l. [DOI] [PubMed] [Google Scholar]
- Van Delden C., Foti M., Lew D. P., Krause K. H. Ca2+ and Mg2+ regulation of inositol 1,4,5-triphosphate binding in myeloid cells. J Biol Chem. 1993 Jun 15;268(17):12443–12448. [PubMed] [Google Scholar]
- Varney M. A., Rivera J., Lopez Bernal A., Watson S. P. Are there subtypes of the inositol 1,4,5-trisphosphate receptor? Biochem J. 1990 Jul 1;269(1):211–216. doi: 10.1042/bj2690211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J., Benevolensky D. Inositol 1,4,5-trisphosphate-induced calcium release from canine aortic sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1987 Dec 10;931(3):354–363. doi: 10.1016/0167-4889(87)90227-8. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]