Abstract
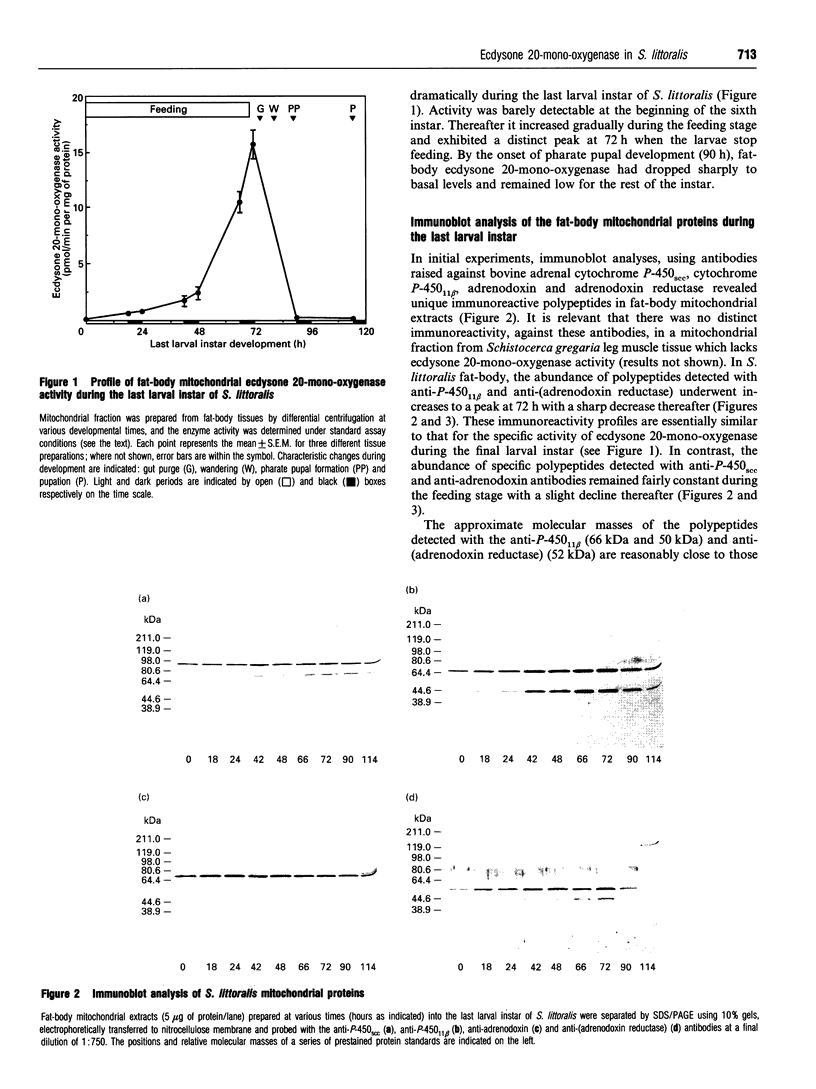
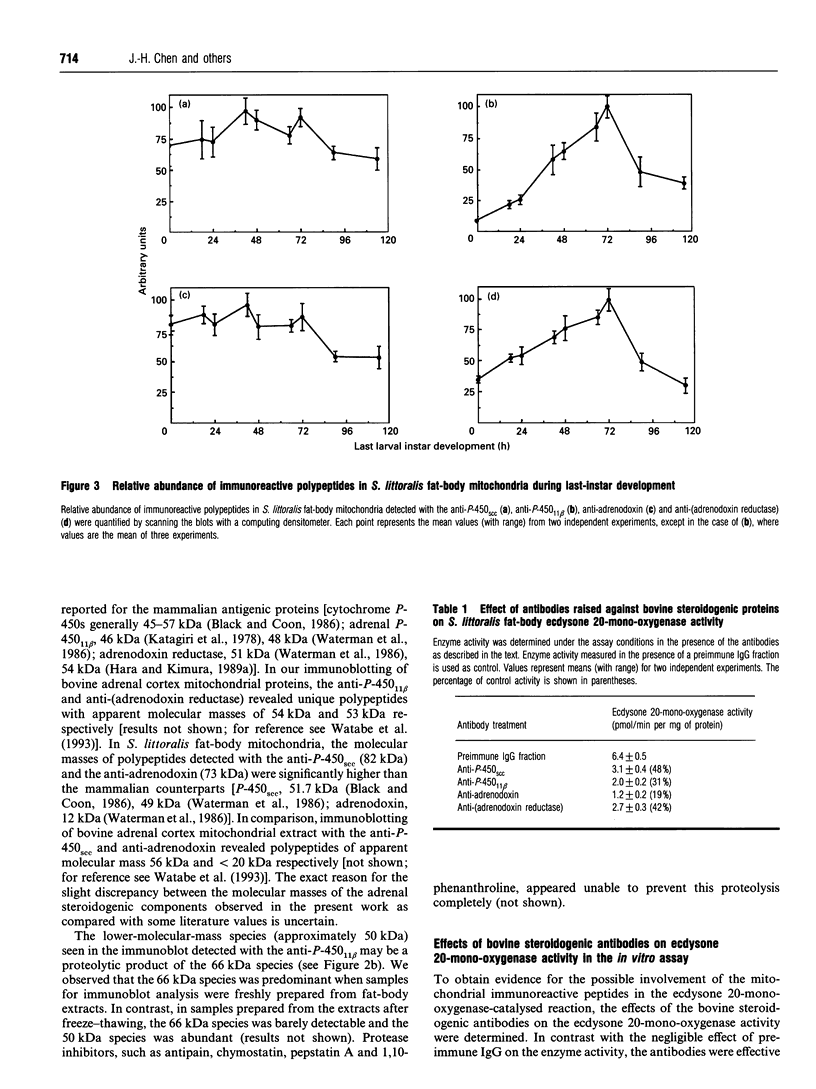
The developmental changes in ecdysone 20-mono-oxygenase during the sixth larval instar of the cotton leafworm, Spodoptera littoralis, were investigated. The specific activity of mitochondrial ecdysone 20-mono-oxygenase in the fat-body exhibited a distinct peak at 72 h, at which time the larvae stop feeding. Immunoblot analyses, using antibodies raised against components of vertebrate mitochondrial steroidogenic enzyme systems [anti-(cytochrome P-450scc), anti-(cytochrome P-450(11) beta), anti-adrenodoxin and anti-(adrenodoxin reductase) antibodies], revealed the presence of specific immunoreactive polypeptides in fat-body mitochondrial extracts. In addition, these antibodies effectively inhibited fat-body mitochondrial ecdysone 20-mono-oxygenase activity. This suggests that the S. littoralis steroid-hydroxylating system(s) may contain polypeptide components analogous to those present in vertebrates. A close correlation between developmental changes in mitochondrial ecdysone 20-mono-oxygenase activity and the abundance of polypeptides (approx. 66 kDa and 50 kDa) recognized by the anti-(cytochrome P-450(11) beta) antibody and a polypeptide (approx. 52 kDa) recognized by the anti-(adrenodoxin reductase) antibody were observed in both fat-body and midgut. These results suggest that developmental changes in the abundance of components of the ecdysone 20-mono-oxygenase system may play an important role in the developmental regulation of the enzyme expression and, hence, of 20-hydroxyecdysone titre.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addya S., Zheng Y. M., Shayiq R. M., Fan J. Y., Avadhani N. G. Characterization of a female-specific hepatic mitochondrial cytochrome P-450 whose steady-state level is modulated by testosterone. Biochemistry. 1991 Aug 27;30(34):8323–8330. doi: 10.1021/bi00098a007. [DOI] [PubMed] [Google Scholar]
- Aflalo L., Meidan R. The hormonal regulation of cholesterol side-chain cleavage cytochrome P450, adrenodoxin, and their messenger ribonucleic acid expression in bovine small-like and large-like luteal cells: relationship with progesterone production. Endocrinology. 1993 Jan;132(1):410–416. doi: 10.1210/endo.132.1.8380385. [DOI] [PubMed] [Google Scholar]
- Bradfield J. Y., Lee Y. H., Keeley L. L. Cytochrome P450 family 4 in a cockroach: molecular cloning and regulation by regulation by hypertrehalosemic hormone. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4558–4562. doi: 10.1073/pnas.88.10.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon M. J., Ding X. X., Pernecky S. J., Vaz A. D. Cytochrome P450: progress and predictions. FASEB J. 1992 Jan 6;6(2):669–673. doi: 10.1096/fasebj.6.2.1537454. [DOI] [PubMed] [Google Scholar]
- Feyereisen R., Durst F. Development of microsomal cytochrome P-450 monooxygenases during the last larval instar of the locust, Locusta migratoria: correlation with the hemolymph 20-hydroxyecdysone titer. Mol Cell Endocrinol. 1980 Nov;20(2):157–169. doi: 10.1016/0303-7207(80)90079-9. [DOI] [PubMed] [Google Scholar]
- Feyereisen R., Koener J. F., Farnsworth D. E., Nebert D. W. Isolation and sequence of cDNA encoding a cytochrome P-450 from an insecticide-resistant strain of the house fly, Musca domestica. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1465–1469. doi: 10.1073/pnas.86.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R., Varak E., Goldberg M. L. Molecular analysis of a cytochrome P450 gene of family 4 on the Drosophila X chromosome. DNA Cell Biol. 1992 Jun;11(5):397–404. doi: 10.1089/dna.1992.11.397. [DOI] [PubMed] [Google Scholar]
- Hara T., Kimura T. Active complex between adrenodoxin reductase and adrenodoxin in the cytochrome P-450scc reduction reaction. J Biochem. 1989 Apr;105(4):601–605. doi: 10.1093/oxfordjournals.jbchem.a122711. [DOI] [PubMed] [Google Scholar]
- Hara T., Kimura T. Purification and catalytic properties of a cross-linked complex between adrenodoxin reductase and adrenodoxin. J Biochem. 1989 Apr;105(4):594–600. doi: 10.1093/oxfordjournals.jbchem.a122710. [DOI] [PubMed] [Google Scholar]
- Imaoka S., Fujita S., Funae Y. Age-dependent expression of cytochrome P-450s in rat liver. Biochim Biophys Acta. 1991 Oct 21;1097(3):187–192. doi: 10.1016/0925-4439(91)90034-7. [DOI] [PubMed] [Google Scholar]
- John M. E., John M. C., Simpson E. R., Waterman M. R. Regulation of cytochrome P-45011 beta gene expression by adrenocorticotropin. J Biol Chem. 1985 May 10;260(9):5760–5767. [PubMed] [Google Scholar]
- Johnson P., Rees H. H. The mechanism of C-20 hydroxylation of alpha-ecdysone in the desert locust, Schistocerca gregaria. Biochem J. 1977 Dec 15;168(3):513–520. doi: 10.1042/bj1680513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M., Takemori S., Itagaki E., Suhara K. Purification of adrenal cytochrome P-450 (cholesterol desmolase and steroid 11beta- and 18-hydroxylase). Methods Enzymol. 1978;52:124–132. doi: 10.1016/s0076-6879(78)52014-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller W. L. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988 Aug;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Mitchell M. J., Smith S. L. Ecdysone 20-monooxygenase activity throughout the life cycle of Drosophila melanogaster. Gen Comp Endocrinol. 1988 Dec;72(3):467–470. doi: 10.1016/0016-6480(88)90170-0. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Nigg H. N., Svoboda J. A., Thompson M. J., Dutky S. R., Kaplanis J. N., Robbins W. E. Ecdysome 20-hydroxylase from the midgut of the tobacco hornworm (Manduca sexta L.). Experientia. 1976 Apr 15;32(4):438–439. doi: 10.1007/BF01920781. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Lee S. S. Tissue distribution of microsomal cytochrome P-450 monooxygenases and their inducibility by phenobarbital in the insecticide resistant LPR strain of house fly, Musca domestica L. Insect Biochem Mol Biol. 1993 Sep;23(6):729–738. doi: 10.1016/0965-1748(93)90047-v. [DOI] [PubMed] [Google Scholar]
- Smith S. L., Bollenbacher W. E., Gilbert L. I. Ecdysone 20-monooxygenase activity during larval-pupal development of Manduca sexta. Mol Cell Endocrinol. 1983 Aug;31(2-3):227–251. doi: 10.1016/0303-7207(83)90151-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe S., Hara T., Kohno H., Hiroi T., Yago N., Nakazawa T. In vitro degradation of mitochondrial proteins by ATP-dependent protease in bovine adrenal cortex. J Biochem. 1993 Jun;113(6):672–676. doi: 10.1093/oxfordjournals.jbchem.a124101. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Zelhof A. C., Shaw B. J., Ch'ang L. Y. Possible involvement of the long terminal repeat of transposable element 17.6 in regulating expression of an insecticide resistance-associated P450 gene in Drosophila. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4855–4859. doi: 10.1073/pnas.89.11.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]