Abstract
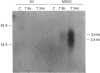
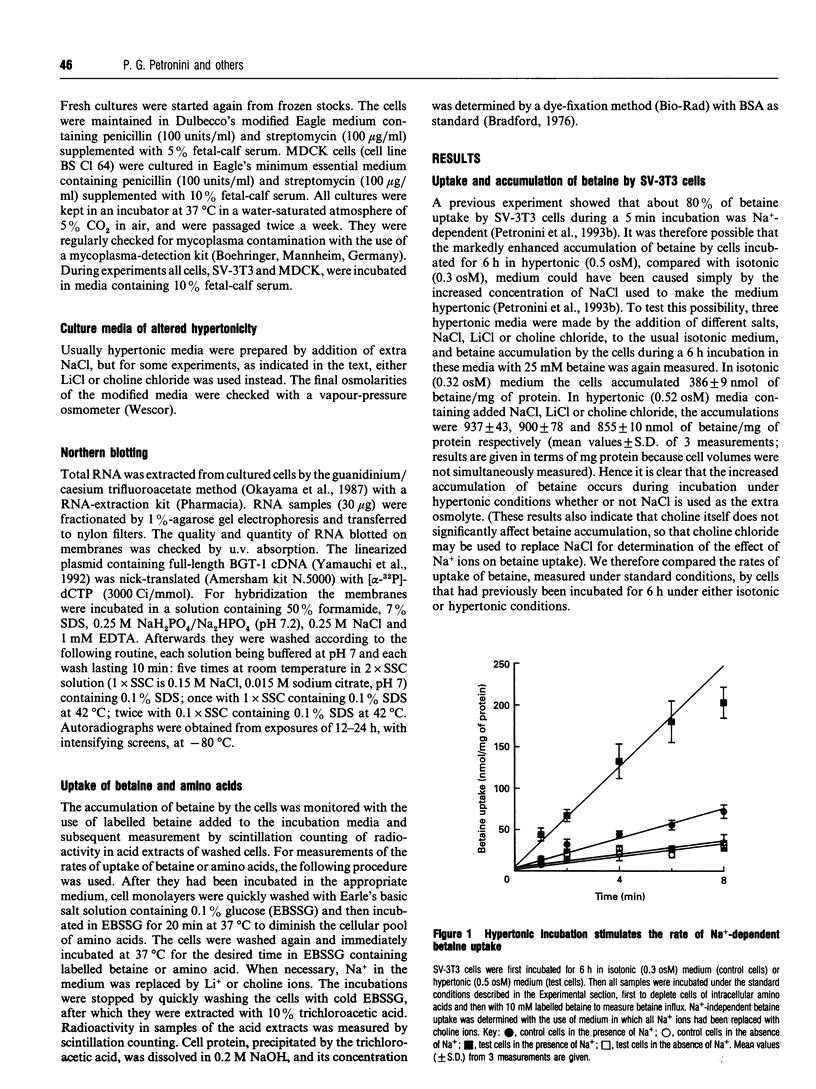
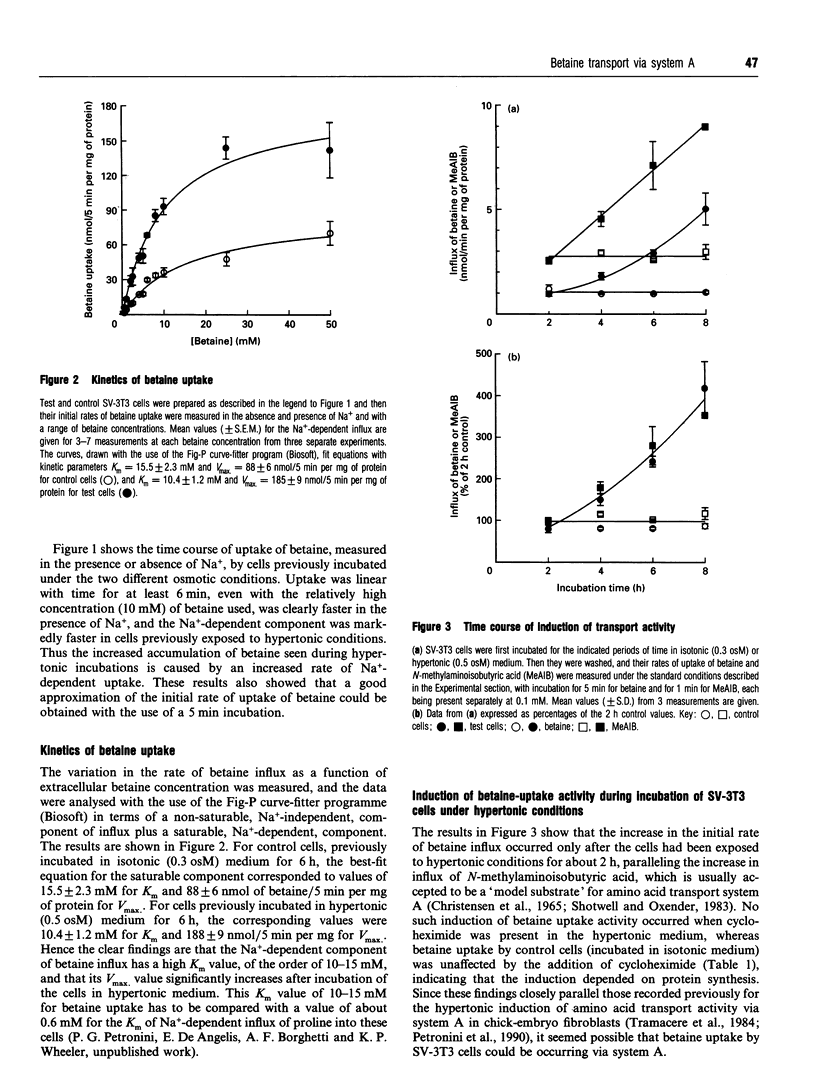
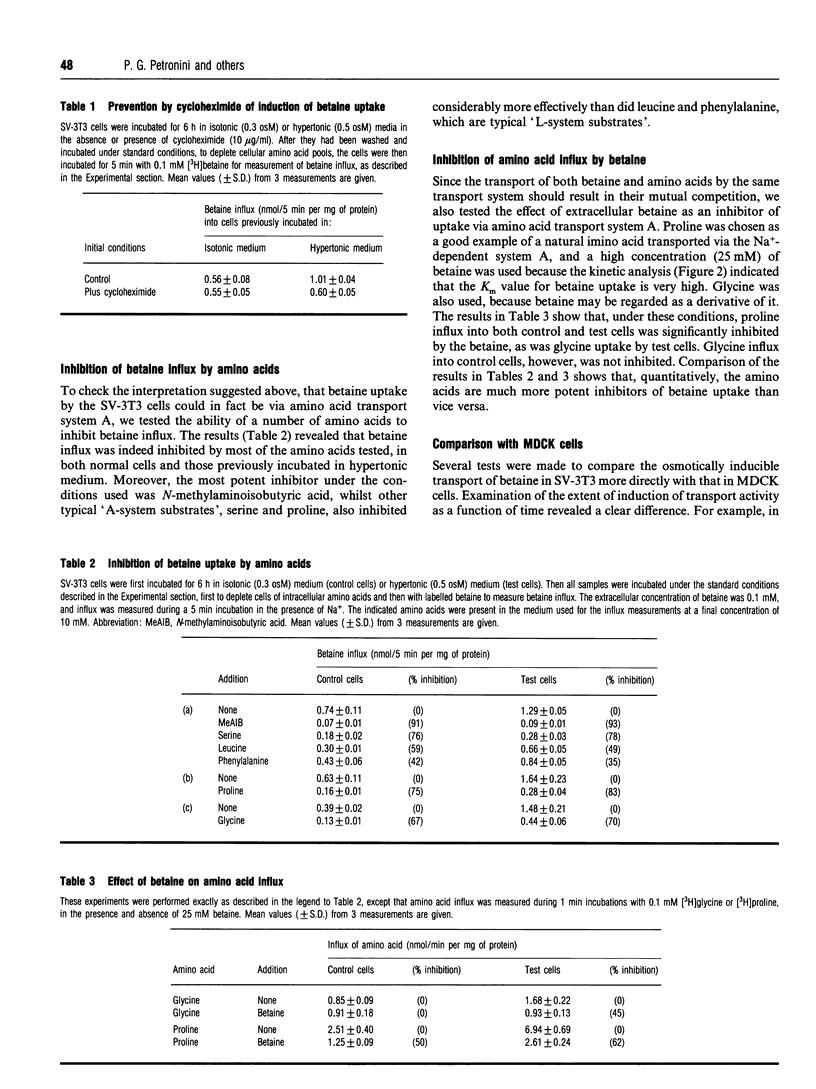
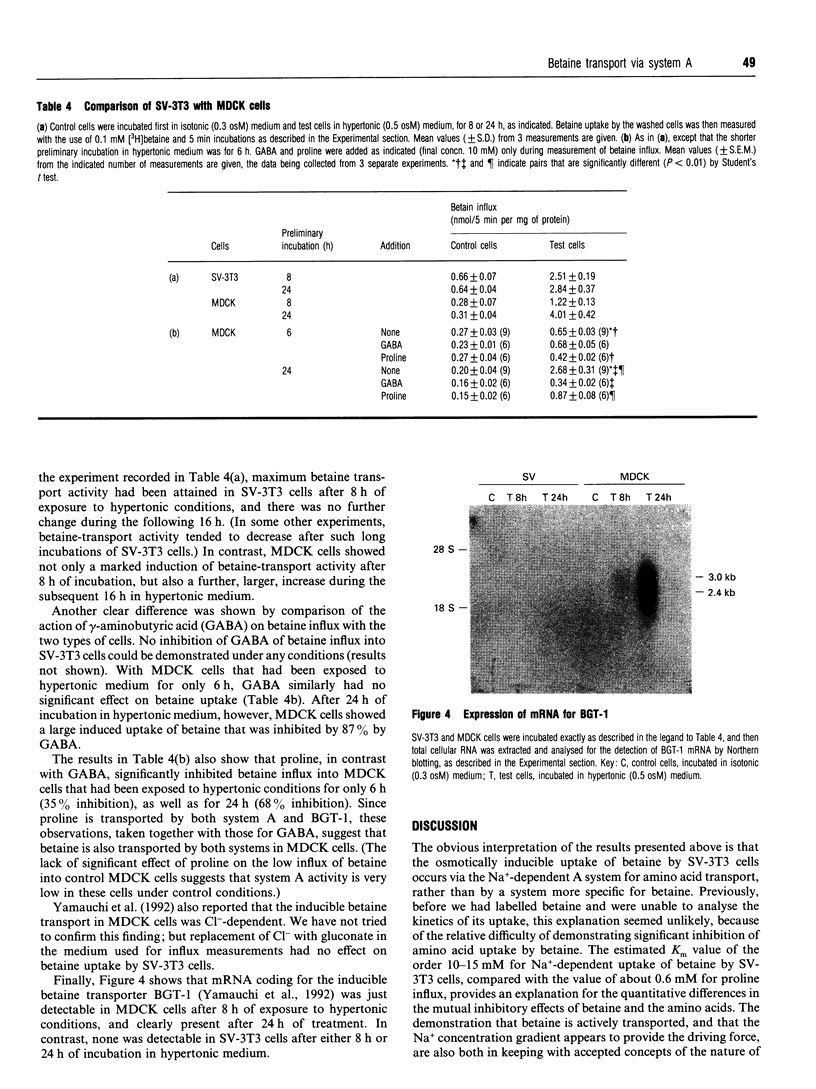
The osmotically inducible uptake of betaine (NNN-trimethylglycine) by SV-3T3 cells has been studied and compared with the similar process in MDCK cells. Betaine uptake by SV-3T3 cells could be described in terms of a saturable, Na(+)-dependent, component plus a small non-saturable, Na(+)-independent, component. Transport was active, producing considerable accumulation of betaine in the cells. After exposure of the cells to hypertonic conditions for 6 h, there was a marked increase in betaine uptake. Kinetic analysis indicated that this increase resulted from an increase in the Vmax. value of the saturable component, from about 88 to 185 nmol of betaine/5 min per mg of protein, the corresponding Km values of about 15 and 10 mM not being significantly different. This induction of transport activity was detectable only after about 2 h exposure of the cells to hypertonic medium, closely paralleling an induction of influx of N-methylaminoisobutyric acid, and was prevented by the presence of cycloheximide. Betaine influx was markedly inhibited by several neutral amino acids, particularly those transported by system A, such as N-methylaminoisobutyric acid and the imino acid proline. A high concentration (25 mM) of betaine also significantly inhibited the uptake of proline by SV-3T3 cells. Although very similar results were obtained with MDCK cells, prolonged exposure of cells to hypertonic conditions revealed distinct differences. When the hypertonic incubation was extended from 6 h to 24 h, betaine transport in SV-3T3 cells either remained the same or decreased, whereas it showed a further marked increase in MDCK cells, and also became sensitive to inhibition by gamma-aminobutyric acid. mRNA for the betaine transporter BGT-1 [Yamauchi, Uchida, Kwon, Preston, Brooks Robey, Garcia-Perez, Burg and Handler (1992) J. Biol. Chem. 267, 649-652] was detectable in MDCK cells exposed to hypertonic medium for 24 h, but not in SV-3T3 cells under any conditions. It is concluded that SV-3T3 cells do not produce a specific inducible transporter analogous to BGT-1, but they can accumulate betaine via the amino acid transport system A.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borghetti A. F., Kay J. E., Wheeler K. P. Enhanced transport of natural amino acids after activation of pig lymphocytes. Biochem J. 1979 Jul 15;182(1):27–32. doi: 10.1042/bj1820027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Oxender D. L., Liang M., Vatz K. A. The use of N-methylation to direct route of mediated transport of amino acids. J Biol Chem. 1965 Sep;240(9):3609–3616. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Turner R. J., Burg M. B. Osmoregulation of betaine transport in mammalian renal medullary cells. Am J Physiol. 1990 Apr;258(4 Pt 2):F1061–F1067. doi: 10.1152/ajprenal.1990.258.4.F1061. [DOI] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Petronini P. G., De Angelis E. M., Borghetti P., Borghetti A. F., Wheeler K. P. Modulation by betaine of cellular responses to osmotic stress. Biochem J. 1992 Feb 15;282(Pt 1):69–73. doi: 10.1042/bj2820069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronini P. G., Tramacere M., Kay J. E., Borghetti A. F. Adaptive response of cultured fibroblasts to hyperosmolarity. Exp Cell Res. 1986 Jul;165(1):180–190. doi: 10.1016/0014-4827(86)90542-2. [DOI] [PubMed] [Google Scholar]
- Petronini P. G., Tramacere M., Wheeler K. P., Borghetti A. F. Induction of amino acid transport activity in chick embryo fibroblasts by replacement of extracellular sodium chloride with disaccharide. Biochim Biophys Acta. 1990 Jul 12;1053(2-3):144–150. doi: 10.1016/0167-4889(90)90006-y. [DOI] [PubMed] [Google Scholar]
- Santoro M. M., Liu Y., Khan S. M., Hou L. X., Bolen D. W. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry. 1992 Jun 16;31(23):5278–5283. doi: 10.1021/bi00138a006. [DOI] [PubMed] [Google Scholar]
- Soler C., Felipe A., Casado F. J., McGivan J. D., Pastor-Anglada M. Hyperosmolarity leads to an increase in derepressed system A activity in the renal epithelial cell line NBL-1. Biochem J. 1993 Feb 1;289(Pt 3):653–658. doi: 10.1042/bj2890653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Kwon H. M., Preston A. S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992 Jan 5;267(1):649–652. [PubMed] [Google Scholar]