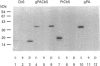
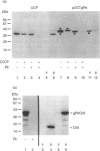
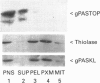
Abstract
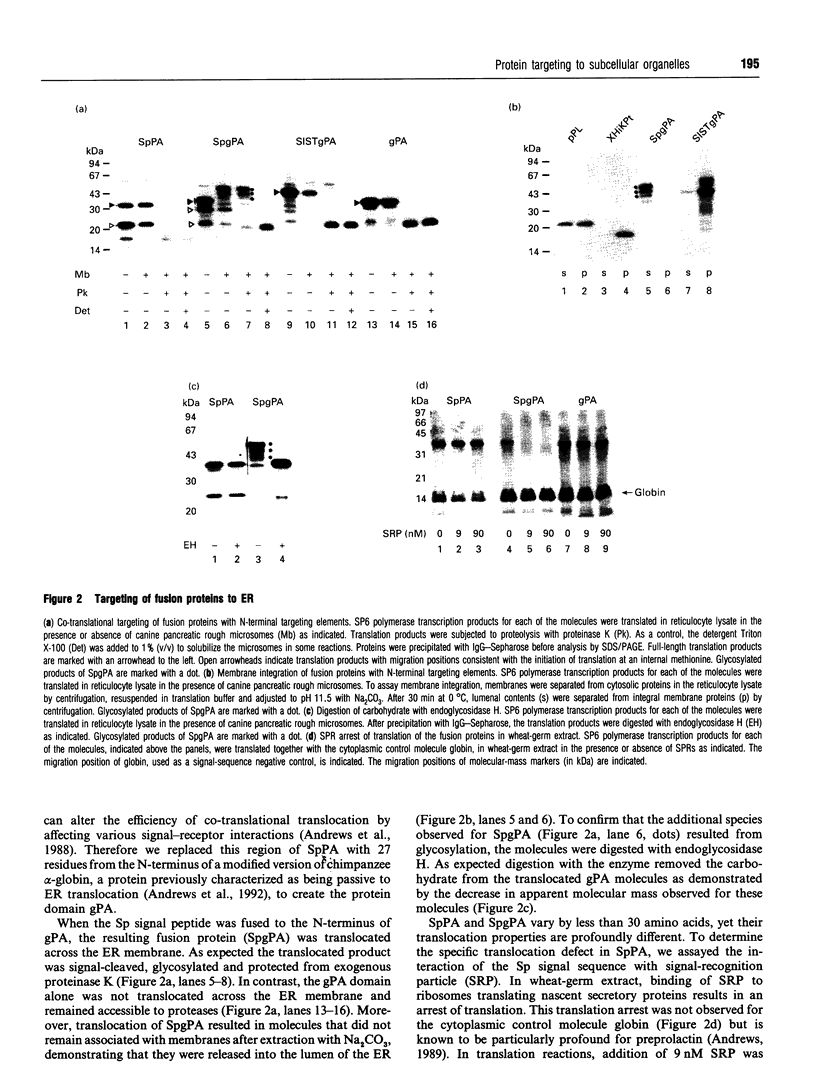
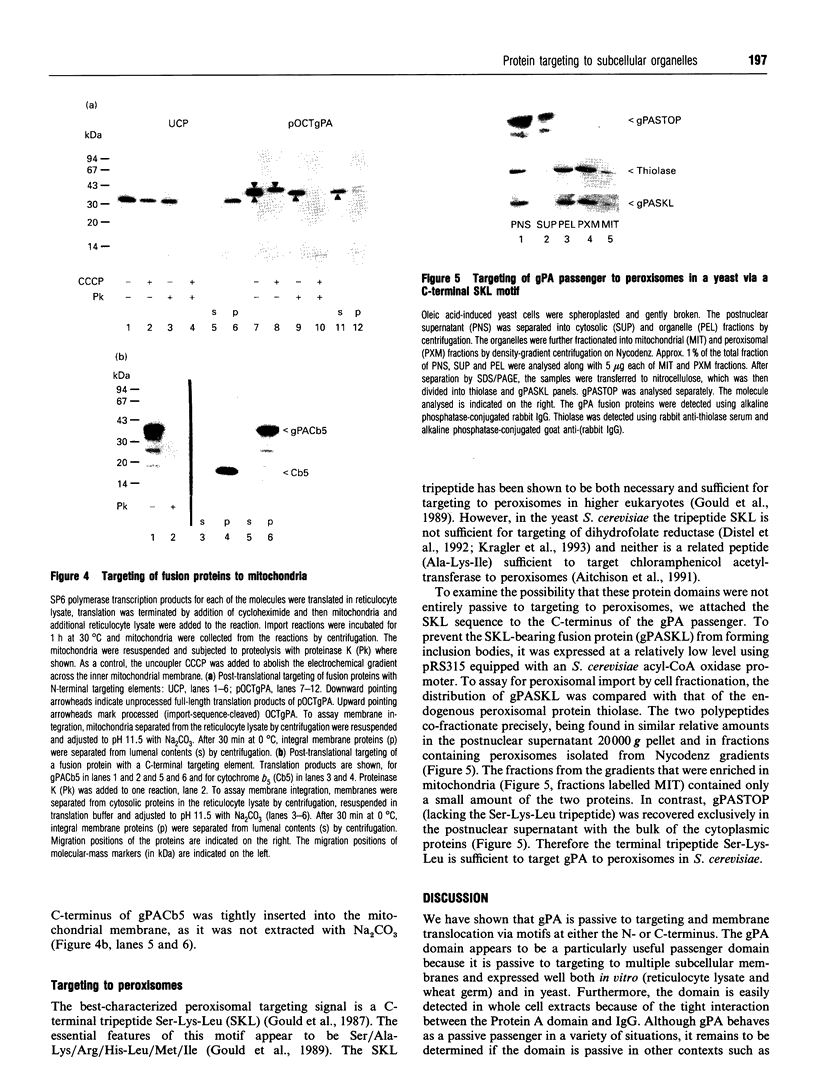
The role of passenger domains in protein targeting was examined by fusing previously characterized targeting motifs to different protein sequences. To compare the targeting requirements for a variety of subcellular compartments, targeting of the fusion proteins was examined for endoplasmic reticulum, mitochondria and peroxisomes in vitro and in yeast. Although most passenger domains were only partially passive to translocation, motif-dependent targeting via motifs positioned at either end of one passenger domain (gPA) was demonstrated for all of the subcellular compartments tested. The data presented extend earlier suggestions that translocation competence is an intrinsic property of the passenger protein. However, the properties that determine protein targeting are not mutually exclusive for the compartments tested. Therefore, although the primary determinant of specificity is the targeting motif, our results suggest that translocation competence of the targeted protein augments the fidelity of transport.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. W., Gupta J., Abisdris G. Evidence that the middle T antigen of polyomavirus interacts with the membrane skeleton. Mol Cell Biol. 1993 Aug;13(8):4703–4713. doi: 10.1128/mcb.13.8.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. W., Perara E., Lesser C., Lingappa V. R. Sequences beyond the cleavage site influence signal peptide function. J Biol Chem. 1988 Oct 25;263(30):15791–15798. [PubMed] [Google Scholar]
- Andrews D. W., Young J. C., Mirels L. F., Czarnota G. J. The role of the N region in signal sequence and signal-anchor function. J Biol Chem. 1992 Apr 15;267(11):7761–7769. [PubMed] [Google Scholar]
- Andrews D. Examining protein translocation in cell-free systems and microinjected Xenopus oocytes. Biotechniques. 1989 Oct;7(9):960-2, 964-7. [PubMed] [Google Scholar]
- Argan C., Lusty C. J., Shore G. C. Membrane and cytosolic components affecting transport of the precursor for ornithine carbamyltransferase into mitochondria. J Biol Chem. 1983 Jun 10;258(11):6667–6670. [PubMed] [Google Scholar]
- Arinç E., Rzepecki L. M., Strittmatter P. Topography of the C terminus of cytochrome b5 tightly bound to dimyristoylphosphatidylcholine vesicles. J Biol Chem. 1987 Nov 15;262(32):15563–15567. [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Tadayyon M., Zhang Y. Beta-lactamase as a probe of membrane protein assembly and protein export. Mol Microbiol. 1990 Oct;4(10):1637–1644. doi: 10.1111/j.1365-2958.1990.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Chuck S. L., Yao Z., Blackhart B. D., McCarthy B. J., Lingappa V. R. New variation on the translocation of proteins during early biogenesis of apolipoprotein B. Nature. 1990 Jul 26;346(6282):382–385. doi: 10.1038/346382a0. [DOI] [PubMed] [Google Scholar]
- Derman A. I., Puziss J. W., Bassford P. J., Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993 Mar;12(3):879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990 Aug;15(8):305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- Distel B., Gould S. J., Voorn-Brouwer T., van der Berg M., Tabak H. F., Subramani S. The carboxyl-terminal tripeptide serine-lysine-leucine of firefly luciferase is necessary but not sufficient for peroxisomal import in yeast. New Biol. 1992 Feb;4(2):157–165. [PubMed] [Google Scholar]
- Dmochowska A., Dignard D., Maleszka R., Thomas D. Y. Structure and transcriptional control of the Saccharomyces cerevisiae POX1 gene encoding acyl-coenzyme A oxidase. Gene. 1990 Apr 16;88(2):247–252. doi: 10.1016/0378-1119(90)90038-s. [DOI] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Mertens D., Kunau W. H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D., Andrews D. W. Both the 5' untranslated region and the sequences surrounding the start site contribute to efficient initiation of translation in vitro. Mol Cell Biol. 1991 May;11(5):2656–2664. doi: 10.1128/mcb.11.5.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folz R. J., Gordon J. I. The effects of deleting the propeptide from human preproapolipoprotein A-I on co-translational translocation and signal peptidase processing. J Biol Chem. 1987 Dec 15;262(35):17221–17230. [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987 Dec;105(6 Pt 2):2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Walter P. C-terminal sequences can inhibit the insertion of membrane proteins into the endoplasmic reticulum of Saccharomyces cerevisiae. Mol Cell Biol. 1992 Jan;12(1):276–282. doi: 10.1128/mcb.12.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V. V., Pokrovskaya I. D., Obukhova T. A., Zozulya S. A. Preparative in vitro mRNA synthesis using SP6 and T7 RNA polymerases. Anal Biochem. 1991 Jun;195(2):207–213. doi: 10.1016/0003-2697(91)90318-n. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Flint N., Gough N. M., Dobberstein B. A tripartite structure of the signals that determine protein insertion into the endoplasmic reticulum membrane. J Cell Biol. 1989 Apr;108(4):1227–1236. doi: 10.1083/jcb.108.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W., Mantsch H. H. Structure of cytochrome b5 in solution by Fourier-transform infrared spectroscopy. Biochemistry. 1989 Feb 7;28(3):931–935. doi: 10.1021/bi00429a002. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 1984 Dec 20;3(13):3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Schatz G. A cytosolic protein contains a cryptic mitochondrial targeting signal. Nature. 1987 Feb 5;325(6104):499–503. doi: 10.1038/325499a0. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Botstein D. Efficiency and diversity of protein localization by random signal sequences. Mol Cell Biol. 1990 Jun;10(6):3163–3173. doi: 10.1128/mcb.10.6.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Kragler F., Langeder A., Raupachova J., Binder M., Hartig A. Two independent peroxisomal targeting signals in catalase A of Saccharomyces cerevisiae. J Cell Biol. 1993 Feb;120(3):665–673. doi: 10.1083/jcb.120.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U., Hartmann E., Rapoport T. A. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 1993 Mar;3(3):72–75. doi: 10.1016/0962-8924(93)90066-a. [DOI] [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990 Apr;10(4):1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Chaidez J., Yost C. S., Hedgpeth J. Determinants for protein localization: beta-lactamase signal sequence directs globin across microsomal membranes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):456–460. doi: 10.1073/pnas.81.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre S., Henning U. The role of the mature part of secretory proteins in translocation across the plasma membrane and in regulation of their synthesis in Escherichia coli. Biochimie. 1990 Feb-Mar;72(2-3):157–167. doi: 10.1016/0300-9084(90)90141-3. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Neupert W., Hartl F. U., Craig E. A., Pfanner N. How do polypeptides cross the mitochondrial membranes? Cell. 1990 Nov 2;63(3):447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Shore G. C. Import of hybrid vesicular stomatitis G protein to the mitochondrial inner membrane. J Biol Chem. 1987 Mar 25;262(9):3929–3931. [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara E., Lingappa V. R. A former amino terminal signal sequence engineered to an internal location directs translocation of both flanking protein domains. J Cell Biol. 1985 Dec;101(6):2292–2301. doi: 10.1083/jcb.101.6.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. On remaining cytoplasmic. Biochimie. 1990 Feb-Mar;72(2-3):89–94. doi: 10.1016/0300-9084(90)90133-2. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Patel H. V., Gerber G. E., Morton R. C., Freeman K. B. Complete nucleotide and derived amino acid sequence of cDNA encoding the mitochondrial uncoupling protein of rat brown adipose tissue: lack of a mitochondrial targeting presequence. Nucleic Acids Res. 1986 May 27;14(10):4025–4035. doi: 10.1093/nar/14.10.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. L., Richardson W. D., Smith A. E. The effect of protein context on nuclear location signal function. Cell. 1987 Jul 31;50(3):465–475. doi: 10.1016/0092-8674(87)90500-9. [DOI] [PubMed] [Google Scholar]
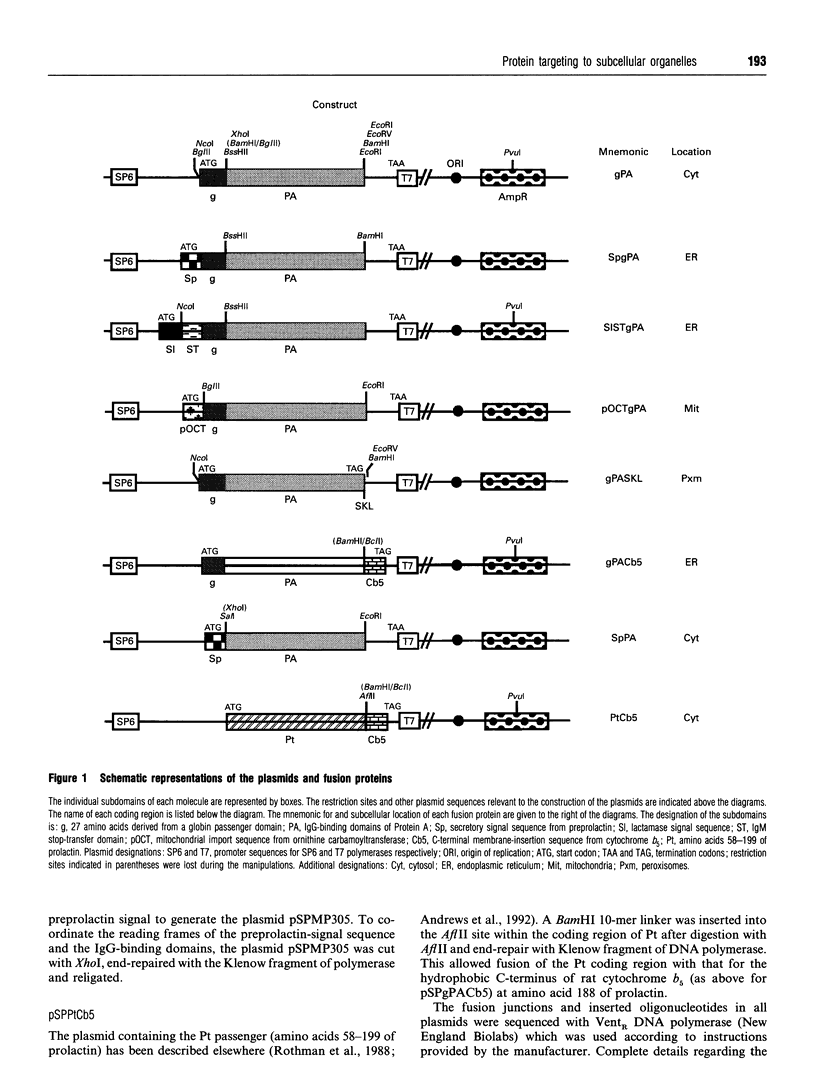
- Rothman R. E., Andrews D. W., Calayag M. C., Lingappa V. R. Construction of defined polytopic integral transmembrane proteins. The role of signal and stop transfer sequence permutations. J Biol Chem. 1988 Jul 25;263(21):10470–10480. [PubMed] [Google Scholar]
- Sanz P., Meyer D. I. Signal recognition particle (SRP) stabilizes the translocation-competent conformation of pre-secretory proteins. EMBO J. 1988 Nov;7(11):3553–3557. doi: 10.1002/j.1460-2075.1988.tb03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silve S., Volland C., Hinnen A., Haguenauer-Tsapis R. In vivo translocation of the cell wall acid phosphatase across the yeast endoplasmic reticulum membrane: are there multiple signals for the targeting process? Biochimie. 1990 Feb-Mar;72(2-3):103–114. doi: 10.1016/0300-9084(90)90135-4. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Radhakrishnan R., Gupta C. M., Khorana H. G. The membrane-embedded segment of cytochrome b5 as studied by cross-linking with photoactivatable phospholipids. J Biol Chem. 1983 Aug 10;258(15):9128–9135. [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Verner K., Lemire B. D. Tight folding of a passenger protein can interfere with the targeting function of a mitochondrial presequence. EMBO J. 1989 May;8(5):1491–1495. doi: 10.1002/j.1460-2075.1989.tb03533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Cytosol-dependent peroxisomal protein import in a permeabilized cell system. J Cell Biol. 1993 Feb;120(3):675–685. doi: 10.1083/jcb.120.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Yost C. S., Hedgpeth J., Lingappa V. R. A stop transfer sequence confers predictable transmembrane orientation to a previously secreted protein in cell-free systems. Cell. 1983 Oct;34(3):759–766. doi: 10.1016/0092-8674(83)90532-9. [DOI] [PubMed] [Google Scholar]
- Yost C. S., Lopez C. D., Prusiner S. B., Myers R. M., Lingappa V. R. Non-hydrophobic extracytoplasmic determinant of stop transfer in the prion protein. Nature. 1990 Feb 15;343(6259):669–672. doi: 10.1038/343669a0. [DOI] [PubMed] [Google Scholar]
- Zerial M., Melancon P., Schneider C., Garoff H. The transmembrane segment of the human transferrin receptor functions as a signal peptide. EMBO J. 1986 Jul;5(7):1543–1550. doi: 10.1002/j.1460-2075.1986.tb04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Wickner W., Dalbey R. E. The cytoplasmic domain of Escherichia coli leader peptidase is a "translocation poison" sequence. Proc Natl Acad Sci U S A. 1988 May;85(10):3363–3366. doi: 10.1073/pnas.85.10.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]