Summary
Background
It is unknown if early COVID-19 monoclonal antibody (mAb) therapy can reduce risk of Long COVID. The mAbs amubarvimab/romlusevimab were previously demonstrated to reduce risk of hospitalization/death by 79%. This study assessed the impact of amubarvimab/romlusevimab on late outcomes, including Long COVID.
Methods
Non-hospitalized high-risk adults within 10 days of COVID-19 symptom onset enrolled in a randomized, double-blind, placebo-controlled phase 2/3 trial of amubarvimab/romlusevimab for COVID-19 treatment. Late symptoms, assessed using a participant-completed symptom diary, were a pre-specified exploratory endpoint. The primary outcome for this analysis was the composite of Long COVID by participant self-report (presence of COVID-19 symptoms as recorded in the diary at week 36) or hospitalization or death by week 36. Inverse probability weighting (IPW) was used to address incomplete outcome ascertainment, giving weighted risk ratios (wRR) comparing amubarvimab/romlusevimab to placebo.
Findings
Participants received amubarvimab/romlusevimab (n = 390) or placebo (n = 390) between January and July 2021. Median age was 49 years, 52% were female, 18% Black/African American, 49% Hispanic/Latino, and 9% COVID-19-vaccinated at entry. At week 36, 103 (13%) had incomplete outcome ascertainment, and 66 (17%) on amubarvimab/romlusevimab and 92 (24%) on placebo met the primary outcome (wRR = 0.70, 95% confidence interval (CI) 0.53–0.93). The difference was driven by fewer hospitalizations/deaths with amubarvimab/romlusevimab (4%) than placebo (13%). Among 652 participants with available diary responses, 53 (16%) on amubarvimab/romlusevimab and 44 (14%) on placebo reported presence of Long COVID.
Interpretation
Amubarvimab/romlusevimab treatment, while highly effective in preventing hospitalizations/deaths, did not reduce risk of Long COVID. Additional interventions are needed to prevent Long COVID.
Funding
National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Amubarvimab and romlusevimab supplied by Brii Biosciences.
Keywords: COVID-19, Monoclonal antibodies, Outpatient treatment, Clinical trial, Post COVID conditions, Long COVID, Post-acute sequelae of SARS-CoV-2 infection (PASC)
Research in context.
Evidence before this study
Published observational and retrospective studies have reported that direct antiviral therapies for COVID-19 (nirmatrelvir/ritonavir, molnupiravir and remdesivir) can reduce the risk of Long COVID, defined variably as the presence of any number of post-acute sequelae at time points between 30 and 180 days of follow-up. In a secondary analysis of a randomized clinical trial of COVID-19 convalescent plasma among outpatients with acute SARS-CoV-2 infection, statistically significant lower odds of post-COVID conditions were observed at day 90 among those receiving treatment within 5 days after symptom onset. A recently published randomized, parallel-group factorial design trial found an approximately 41% relative reduction in the cumulative incidence of Long COVID, defined as participant-reported receipt of a Long COVID diagnosis from a medical provider, by day 300 in the group that received the anti-diabetes drug metformin compared to participants that received placebo for metformin (some participants in both arms also received fluvoxamine or ivermectin). On November 10, 2023, we conducted a search on PubMed using the keywords “COVID-19,” “monoclonal antibody,” and “clinical trial.” This search did not yield any randomized, placebo-controlled trials that investigated the impact of early treatment with monoclonal antibodies for SARS-CoV-2 infection on post-COVID outcomes and compared these outcomes between treatment groups. To date, no placebo-controlled studies have evaluated the effect of early, potent, COVID-19 monoclonal antibody therapy on post-COVID outcomes.
Added value of this study
This is the first randomized, placebo-controlled study to evaluate the effect of early administration of a COVID-19 monoclonal antibody therapy on post-COVID outcomes. Here we demonstrate that a monoclonal antibody therapy that was highly effective in preventing hospitalization and death in non-hospitalized patients at high-risk for COVID-19 progression did not have a meaningful effect on the prevalence of Long COVID or other participant self-reported symptoms at 36 weeks after treatment.
Implications of all the available evidence
Available evidence suggests there may be differential effects of acute COVID-19 therapies on post-COVID outcomes. These effects may differ based on the mechanism of action of the therapeutic agent. However, differences in study design, participant characteristics, definition of post-COVID outcomes/Long COVID, and duration of evaluation across the studies makes direct comparisons challenging. The totality of the evidence suggests that additional randomized, placebo-controlled studies are warranted to determine the benefit of therapies given during acute COVID-19 for Long COVID prevention.
Introduction
Long COVID or post-COVID conditions consist of a wide spectrum of clinical manifestations.1, 2, 3 It is not known if early monoclonal antibody (mAb) therapy prevents Long COVID. Several observational and retrospective studies have investigated the potential for interventions, such as SARS-CoV-2 vaccination or antiviral nirmatrelvir/ritonavir, molnupiravir or remdesivir therapy, to mitigate or prevent Long COVID.4, 5, 6, 7, 8 Secondary analyses of randomized, controlled trials of early administration of metformin and convalescent plasma have reported significant decreases in the incidence of late outcomes.9,10 To date, there have been no peer-reviewed reports on post-acute COVID outcomes from randomized placebo-controlled interventional trials of mAbs in outpatients.
We evaluated the anti-SARS-CoV-2 mAbs amubarvimab/romlusevimab (Brii Biosciences, Tsinghua University and 3rd People's Hospital of Shenzhen) in a phase 2/3 randomized, blinded, placebo-controlled trial within the ACTIV-2/A5041 platform of therapeutics for non-hospitalized adults with acute COVID-19.11, 12, 13 We previously demonstrated that amubarvimab/romlusevimab significantly reduced the risk of hospitalization and/or death through day 28 (79% risk reduction).14 Here, we present results from an exploratory analysis of the impact of treatment of acute COVID-19 with amubarvimab/romlusevimab on late complications through 36 weeks as compared to placebo. Our analysis includes examination of participant-reported Long COVID and hospitalizations/deaths, return to pre-COVID-19 health, and health-related quality of life (HRQOL).
Methods
Study design and participants
ACTIV-2/A5401 is a multicenter, randomized, controlled, phase 2/3 adaptive platform trial designed to evaluate the safety and efficacy of investigational agents for the treatment of non-hospitalized adults with mild-to-moderate COVID-19 (NCT04518410).13 Key exploratory objectives of the trial (integrated into protocol version 6.0—see Supplement) included assessment of persistent symptoms (i.e., Long COVID) and HRQOL beyond the acute COVID-19 period; as these were exploratory objectives they were not included in the Primary Statistical Analysis Plan (SAP), but rather in a Long COVID SAP prior to undertaking the analysis. The protocol was approved by a central institutional review board (IRB), Advarra (Pro00045266), for United States (US) sites with additional local IRB review and approval as required by sites, and by local Ethics Committees for non-US sites. All participants provided written informed consent.
Participants who were enrolled in the phase 2/3 evaluation of amubarvimab/romlusevimab (and started treatment) were included in the analysis. Eligibility included outpatient adults (≥18 years) at high risk of progression to severe COVID-19 (defined in Supplemental Appendix) with a positive upper respiratory tract SARS-CoV-2 molecular or antigen test and expected to initiate study treatment within 10 days (reduced to 7 days during the trial) of symptom onset. Complete eligibility criteria have been described previously.14
Randomization and masking
Participants were randomized 1:1 by a web-based interactive response system to amubarvimab/romlusevimab or blinded placebo, with stratification by days of symptoms at enrollment (≤ or >5 days) using permuted blocks.
Procedures
Study intervention was administered as sequential intravenous (IV) infusions of 1000 mg of amubarvimab followed by 1000 mg of romlusevimab, or equivalent volumes of saline placebo (or, for a single participant, placebo for another agent evaluated on the platform) on study day 0.15
Assessments
Participant-completed symptom diaries
Two symptom diaries were self-administered by participants during the study. The acute symptom diary was completed daily from enrollment (day 0) through day 28 with each of 13 viral illness symptoms graded as “absent”, “mild”, “moderate” or “severe”.16 Participants reported the worst severity over the preceding 24 h. The long-term symptom diary was administered at weeks 12, 24, 36, 48, and 72, and was developed partway through the study after enrollment had been initiated. The long-term diary included grading of the same 13 symptoms from the acute symptom diary plus 14 Long-COVID-related symptoms chosen based on available literature at the time17 (Supplementary Appendix). In the long-term diary, participants reported the “overall” severity of each symptom over the preceding 4 weeks. The long-term diary also included two-COVID-specific global assessments asking participants to report: 1) the overall severity of their COVID-19 symptoms over the previous 4 weeks, reported as “no symptoms”, “mild”, “moderate” or “severe”, and 2) whether they had returned to their usual (pre-COVID) health at the time of diary completion, “yes” or “no”.
This exploratory analysis focuses on outcomes from the long-term symptom diary completed at 36 weeks after treatment due to incomplete data at earlier time points caused by diary implementation after the study had begun and at later time points as participants were continuing long-term follow-up at the time of the analysis. Total symptom scores were calculated for day 0 and separately for week 36 by summing the individual symptom scores, with absent scored as 0, mild as 1, moderate as 2, and severe as 3. Thus, with 13 individual symptoms assessed on day 0, the total possible symptom score ranged from 0 to 39. At week 36, with 27 individual symptoms assessed, the total possible symptom score ranged from 0 to 81. Global assessment responses were not included in total symptom score calculation.
Participant completed HRQOL questionnaires
Participants also completed two HRQOL questionnaires, the EQ-5D-5L and the Medical Outcomes Study 36-Item Short Form Health Survey Version 2 (SF-36v2), on the same schedule as the long-term diary.18,19 In the EQ-5D-5L questionnaire, participants were asked to report their degree of perceived problems (none, slight, moderate, severe, or extreme) in five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, and to rate their health on a vertical visual analogue scale (VAS) ranging from 0 (“the worst health you can imagine”) to 100 (“the best health you can imagine”). The SF-36v2 assessed eight health domains: physical functioning, physical role, bodily pain, general health perceptions, vitality, social role functioning, emotional role functioning, and mental health.
Outcome measures
The primary outcome for this exploratory analysis was the composite of all-cause hospitalization or death at or prior to week 36 or presence of Long COVID at week 36, defined as presence of COVID-19 symptoms of any severity within the prior 4 weeks in response to the first global assessment question in the long-term diary. This composite outcome was selected to retain the randomized population for comparative analysis of treatment effects. The definition of Long COVID is supported by our prior work demonstrating the internal validity of this definition, including a higher frequency of multiple individual Long COVID symptoms among participants with versus without Long COVID by this definition (also see eFigures S6–S8).20
Secondary outcome measures included, at week 36: the composite of hospitalization or death; the composite of death or Long COVID; severity of Long COVID; the composite of not returning to usual (pre-COVID-19) health (global assessment question) or death; the number of individual symptoms reported (of possible 27); total symptom score across the 27 symptoms (range 0–81); and worst symptom severity reported among the 27 symptoms. The 27 individual symptoms were also grouped a priori into eight categories: upper respiratory, cardiopulmonary/cardiothoracic, constitutional, musculoskeletal, gastrointestinal, neurocognitive, sensory, and dermatologic (eTable 1). HRQOL outcomes included the presence of any problems for each EQ-5D-5L domain, EQ-5D-5L VAS score, and scores for each SF-36v2 domain.
Statistical analysis
The analysis population consisted of all participants who were randomized and received amubarvimab/romlusevimab or placebo, with exclusion of participants enrolled at six study sites where data integrity was a concern. Comparisons were by regimen received. The primary outcome was estimated and compared between arms using modified Poisson regression with log-link and robust variance to obtain a risk ratio.21 There was a similar proportion of participants meeting the primary outcome as there were with incomplete results, and thus restricting the analysis to those with complete data could introduce bias. To help mitigate potential selection bias, inverse probability weighting (IPW) was used.22,23 Each participant with an observed outcome received a weight inversely proportional to the estimated probability of not being censored (due to loss to follow-up or having a missing diary entry). Three sets of weights were computed using logistic regression models fitted within each treatment arm: Model 1 assumed non-informative censoring by including indicator variables for time interval (Day 0–28 and Day 28–Week 36) and if the diary was completed at week 36; Model 2 expanded the first model to allow for dependence of censoring on baseline covariates (age, sex, race, body mass index (BMI), number of high risk comorbidities, symptom duration at study entry (days), and day 0 symptom score) and their interactions with the indicator variables included in Model 1; and Model 3 expanded the second model to include an indicator for whether symptoms were present at days 22–28 and the associated interaction term (see Supplementary Methods for further details). The weights were then used in planned regression models to account for incomplete ascertainment when calculating the risk ratios. Results using the weights from Model 3 were considered the main analysis and are reported in the text (results using Models 1 and 2 were similar and are also shown in tables/figures). As a sensitivity analysis, a complete case type of analysis was undertaken (see Supplement).
Supportive analyses not including hospitalization prior to week 36 in the outcome (because Long COVID is possible after hospitalization) compared the proportion 1) with the composite of death or Long COVID, and 2) with the composite of not returned to usual health or death, using the same modified Poisson regression approach, based on observed outcomes and IPW analysis (adaptations of Model 3 was used; see Supplementary Methods). The proportion hospitalized or died through week 36 was compared between arms using ratio of proportions, estimated from Kaplan–Meier methods. Other secondary analyses were restricted to those with observed data and included comparisons of clinical outcome severity (categories: none, mild, moderate, severe, hospitalized/died) and worst symptom severity (among 27 individual symptoms) using cumulative logistic regression and Wald test, comparisons of dichotomous outcomes including presence of symptoms in each symptom category (upper respiratory, cardiopulmonary/cardiothoracic, constitutional, musculoskeletal, gastrointestinal, neurocognitive, sensory, and dermatologic) and problems per EQ-5D-5L domains using modified Poisson regression, and comparisons of distributions of quantitative outcomes, including number of symptoms, total symptom score, VAS score and SF-36v2 scores using non-parametric Wilcoxon rank sum tests; see Supplemental Methods for a summary of outcomes and corresponding statistical methods. All analyses used two-sided 5% significance level without adjustment for multiple comparisons. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Role of the funding source
The funding source had representatives on the study team and were involved in protocol development, study conduct, and analysis of the data.
Results
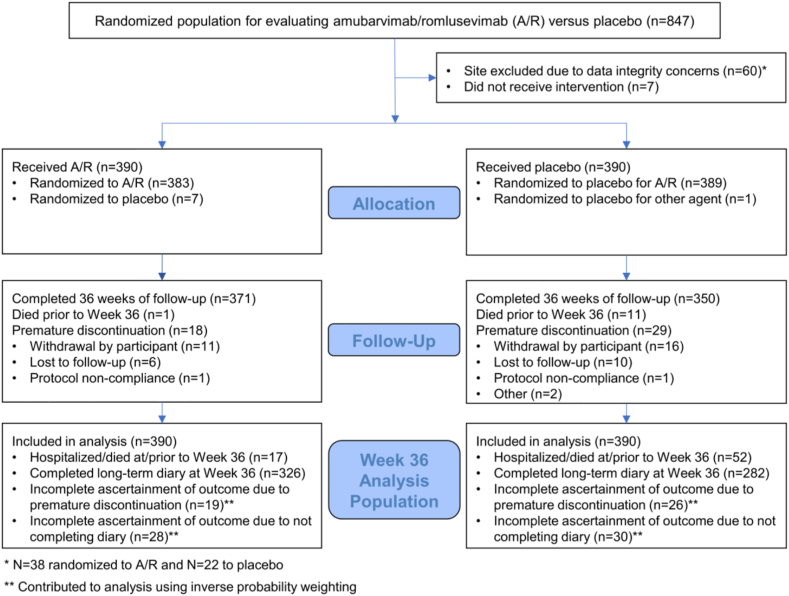
Eight-hundred forty-seven were randomized from January 2021 to July 2021 (prior to emergence of omicron variants) from sites in the US, Argentina, Brazil, Mexico, Philippines, and South Africa, of which 780 participants met criteria for inclusion in the analysis, 390 receiving amubarvimab/romlusevimab and 390 receiving placebo (Fig. 1 and Table 1). Median age was 49 years, 52% were female sex, >99% cis-gender, 18% Black/African American, and 49% Hispanic/Latino. Median BMI was 29.8 kg/m2, 28% were current cigarette smokers, 14% were previous smokers, and 94% reported at least 1 high-risk comorbidity. Only 9% had received at least one dose of a COVID-19 vaccine prior to study entry; 28% received at least one dose of a COVID-19 vaccine by week 36. At the end of the acute phase, 56% of participants reported the presence of any targeted symptoms in the daily symptom diary during days 22–28 (57% for amubarvimab/romlusevimab and 55% for placebo).
Fig. 1.
Consort diagram.
Table 1.
Baseline characteristics prior to receiving amubarvimab/romlusevimab (A/R) or placebo.
| A/R (n = 390) | Placebo (n = 390) | Total (n = 780) | |
|---|---|---|---|
| Country of enrollment | |||
| United States | 251 (64%) | 261 (67%) | 512 (66%) |
| South Africa | 72 (18%) | 59 (15%) | 131 (17%) |
| Argentina | 49 (13%) | 49 (13%) | 98 (13%) |
| Brazil | 15 (4%) | 18 (5%) | 33 (4%) |
| Mexico | 3 (1%) | 2 (1%) | 5 (1%) |
| Philippines | 0 (0%) | 1 (<1%) | 1 (<1%) |
| Age, median (quartiles) years | 48 (39, 58) | 49 (39, 59) | 49 (39, 58) |
| Female sex, n (%) | 200 (51%) | 206 (53%) | 406 (52%) |
| Cis-gender, n (%) | 390 (100%) | 388 (99%) | 778 (>99%) |
| Race, n (%) | |||
| White | 275 (71%) | 289 (74%) | 564 (72%) |
| Black or African American | 80 (21%) | 61 (16%) | 141 (18%) |
| Asian | 16 (4%) | 21 (5%) | 37 (5%) |
| American Indian or Alaska Native | 0 (0%) | 1 (<1%) | 1 (<1%) |
| Native Hawaiian or Other Pacific Islander | 1 (<1%) | 1 (<1%) | 2 (<1%) |
| Multiple | 4 (1%) | 2 (1%) | 6 (1%) |
| Other | 14 (4%) | 15 (4%) | 29 (4%) |
| Hispanic/Latino ethnicity, n (%) | 192 (49%) | 190 (49%) | 382 (49%) |
| BMI (kg/m2), median (quartiles) | 29.5 (25.8, 35.3) | 30.0 (26.1, 36.1) | 29.8 (26.0, 35.9) |
| Cigarettes smoking statusa | |||
| Current | 105 (27%) | 112 (29%) | 217 (28%) |
| Former | 54 (14%) | 54 (14%) | 108 (14%) |
| Never | 231 (59%) | 223 (57%) | 454 (58%) |
| Reporting >1 high-risk co-morbidity, n (%)b | 364 (93%) | 366 (94%) | 730 (94%) |
| SARS-CoV-2 Vaccination, n (%)c | 30 (8%) | 40 (10%) | 70 (9%) |
Excludes 1 placebo participant with a missing value.
High Risk Comorbidities include active cancer, moderate to severe asthma, chronic kidney disease, chronic liver disease, history of cirrhosis, chronic lung disease, current smoker, cardiovascular disease, diabetes, hypertension, treatment with biologics/immunomodulators/cancer chemotherapy within 90 days of entry, HIV with CD4 count <200 cells/mm³, receiving corticosteroids within 30 days of entry, and obesity.
Defined as having received at least 1 dose of COVID-19 vaccine regardless of brand.
Diary completeness
In total, 652/780 (84%) of participants completed or partially completed the long-term diary at week 36 (amubarvimab/romlusevimab, n = 339 [87%] or placebo, n = 313 [80%]). 128 (16%) participants were missing diary data at week 36, 51 (13%) on amubarvimab/romlusevimab and 77 (20%) on placebo; 12 (2%) participants were missing diary data due to death before week 36 (1 on amubarvimab/romlusevimab and 11 on placebo).
Primary outcome
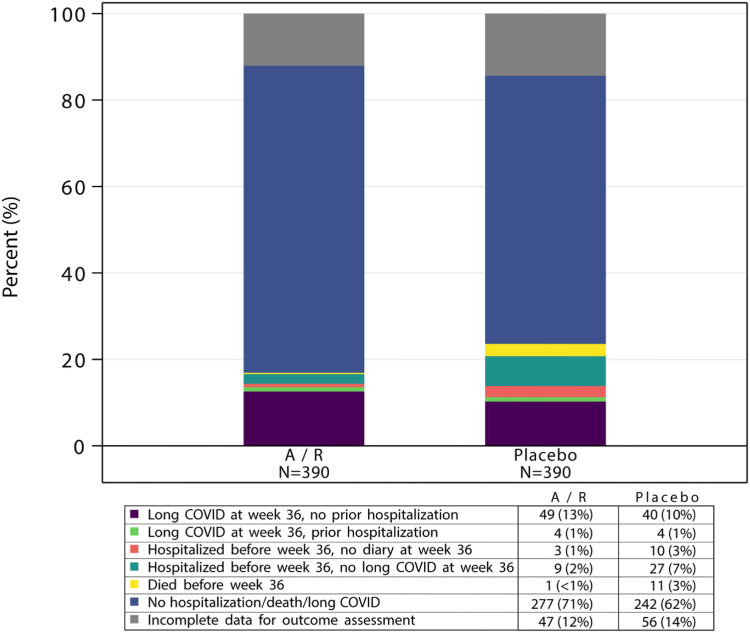
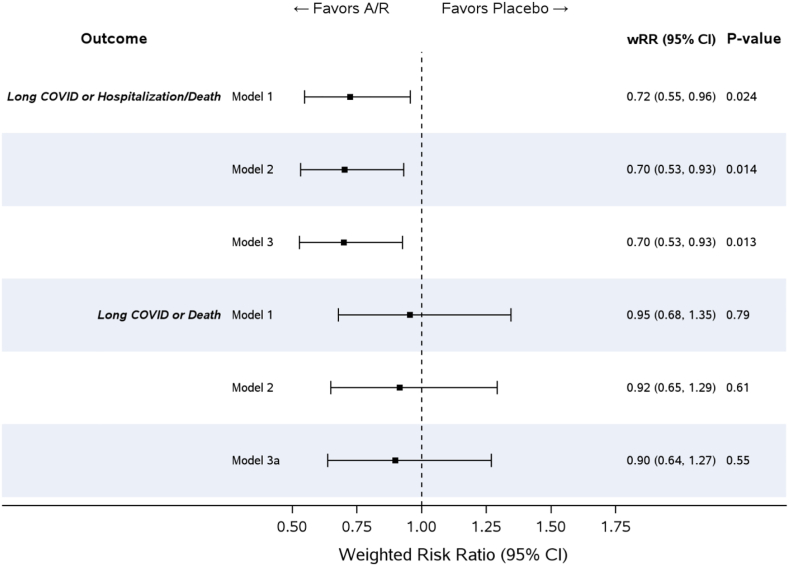
Sixty-six participants (17%) on amubarvimab/romlusevimab and 92 (24%) on placebo met the primary composite outcome of presence of Long COVID at week 36, or hospitalization or death by week 36. Forty-seven (12%) on amubarvimab/romlusevimab and 56 (14%) on placebo had incomplete data for determining the primary outcome (Fig. 2). Based on the weights determined from the IPW Model 3, the weighted Risk Ratio [wRR] for amubarvimab/romlusevimab versus placebo was 0.70; 95% CI: 0.53, 0.93; p = 0.01 (Fig. 3). The difference was driven by fewer hospitalizations/deaths in the amubarvimab/romlusevimab arm (n = 17 [4%]) than in the placebo arm (n = 52 [13%]), with RR for hospitalizations/deaths of 0.33 (95% CI: 0.19, 0.56; p < 0.001; eFigure 1). The majority of hospitalizations occurred before day 28 (9 in amubarvimab/romlusevimab arm and 43 in placebo arm before day 28, and 7 in amubarvimab/romlusevimab arm and 9 in placebo arm after day 28). In total, 12 deaths occurred, including 11 in the placebo arm within the first 28 days (all of which were previously hospitalized) and one in the amubarvimab/romlusevimab arm after day 28, without a previous hospitalization. Examining hospitalization/death and clinical outcome severity of self-reported overall symptoms (Long COVID) at week 36 and hospitalization/death through week 36, these outcomes were worse in the placebo arm (Wald test, p = 0.002, eFigure 2), driven by hospitalization/death differences and not Long COVID symptom severity. Among those with a diary response (n = 652), 53 (16%) on amubarvimab/romlusevimab and 44 (14%) on placebo reported presence of Long COVID.
Fig. 2.
Week 36 long COVID, hospitalizations, and death by amubarvimab/romlusevimab (A/R) versus placebo treatment.
Fig. 3.
Primary and supportive outcome results comparing Amubarvimab/romlusevimab (A/R) versus placebo treatment. Weighted Model 1 = IPW model based on time interval. Weighted Model 2 = IPW model based on time interval, baseline characteristics and their interactions with time interval. Weighted Model 3 = IPW model based on time interval, baseline characteristics, day 22–28 symptom variable, and their interactions with time interval. Weighted Model 3a = IPW model based on time interval, baseline characteristics, day 22–28 symptom variable, whether or not a participant was hospitalized prior to week 36, and their interactions with time interval.
The frequency of the composite of death or Long COVID was similar in the two arms, 54 (14%) on amubarvimab/romlusevimab and 55 (14%) on placebo (wRR = 0.90; 95% CI: 0.64, 1.27; p = 0.55; Model 3a, Fig. 3). In post-hoc analysis, when adjusting for symptom duration strata, results were identical (data not shown).
Conclusions based on complete case type of analyses for both the primary composite Long COVID/hospitalization/death outcome and the Long COVID/death outcome support the IPW analyses (Supplementary Appendix).
Secondary outcomes
Among those with diary responses, 16% in the amubarvimab/romlusevimab arm versus 22% in the placebo group reported not having returned to usual health. The composite of failure to return to health or death favored amubarvimab/romlusevimab (wRR = 0.65, 95% CI: 0.48, 0.89; p = 0.006, eTable 2).
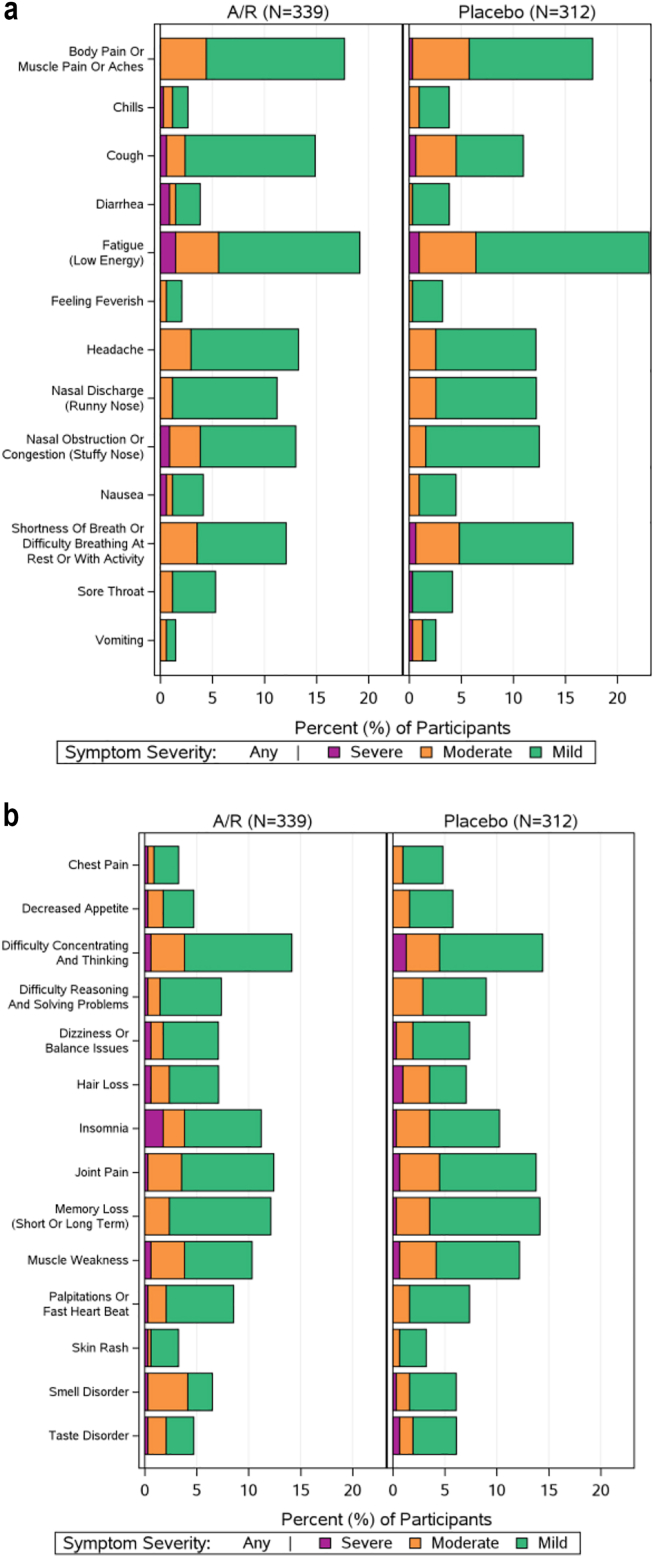
41% and 39% in the amubarvimab/romlusevimab and placebo arms, respectively, reported at least one individual symptom (of 27) as present at week 36, the majority of which were mild. The most commonly reported individual symptoms (reported by ≥15% of all participants) were fatigue (21%) and musculoskeletal pain (18%) (Fig. 4). No significant differences were observed for amubarvimab/romlusevimab versus placebo for the worst symptom score (p = 0.76, eFigure 3), number of symptoms (median [quartiles] 0 [0,3] versus 0 [0,3], p = 0.85, eFigure 4), total symptom score (0 [0,3] versus 0 [0,3] p = 0.83, eFigure 5), or the proportion reporting symptoms in each of the pre-defined symptom categories (p ≥ 0.21 for all comparisons, eTable 3). In post-hoc analysis, no differences were observed for individual symptoms (p ≥ 0.14 for all comparisons, eTable 4).
Fig. 4.
Frequency and severity of (a) 13 viral illness long COVID symptoms and (b) 14 additional long COVID symptoms reported in participant long-term diary at week 36 by participants who received Amubarvimab/romlusevimab (A/R) (n = 339) or Placebo (n = 312).
Health-related quality of life measures: EQ-5D-5L and SF-36v2
The EQ-5D-5L and SF-36v2 questionnaires had similar levels of completeness as the long-term diary. Among those who completed the EQ-5D-5L questionnaire, 26% and 31% in the amubarvimab/romlusevimab and placebo arms, respectively, reported some level of pain or discomfort, RR = 0.83; 95% CI: 0.64, 1.06; p = 0.14; 19% and 17%, reported some level of anxiety/depression, RR = 1.12; 95% CI: 0.80, 1.56; p = 0.51; 13% and 17% reported having some problems doing usual activities, RR = 0.77; 95% CI: 0.53, 1.13; p = 0.18; 10% and 14% reported some level of mobility (walking) problems, RR = 0.71; 95% CI: 0.46, 1.10; p = 0.12; and 3% and 5% reported some problems with self-care (washing or dressing), RR = 0.51; 95% CI: 0.22, 1.19; p = 0.12 (eTable 5). No differences between amubarvimab/romlusevimab and placebo were observed in the VAS health score (median [Q1, Q3] 90 [84, 100] for amubarvimab/romlusevimab versus 90 [80, 100] for placebo; p = 0.16).
Among those who completed the SF-36v2 questionnaire, there were no significant differences between arms in any of the eight health domains (p ≥ 0.21, eTable 6) or overall physical component (median [Q1, Q3] score, 57.9 [52.4, 59.7] amubarvimab/romlusevimab versus 58.4 [50.2, 59.8] placebo, p = 0.72) or mental component (56.8 [49.7, 59.6] versus 57.2 [50.9, 60.2], p = 0.28) summary scores. Overall, 94% of participants reported good, very good, or excellent health, and 90% reported that their health was about the same, somewhat better, or much better than one year ago.
Discussion
In this randomized, double-blind, placebo-controlled phase 2/3 trial of high-risk, non-hospitalized adults with mild-to-moderate COVID-19, we assessed the impact of the combination SARS-CoV-2 mAbs, amubarvimab/romlusevimab, compared to placebo on post-acute COVID outcomes. These protocol-defined assessments were performed within a trial in which amubarvimab/romlusevimab was found to be highly effective in reducing 28-day all-cause hospitalization and death.14 In the primary randomized comparison, we found that amubarvimab/romlusevimab-treated participants had a significantly lower risk of the primary composite outcome of all-cause hospitalization or death at or prior to week 36 or Long COVID at 36 weeks that was driven by fewer hospitalizations/deaths in the amubarvimab/romlusevimab arm. We incorporated hospitalization and death into our primary composite outcome to account for the severity of these events while our supplementary analyses enabled us to specifically explore Long COVID including individual symptoms or symptom clusters as an isolated outcome. No benefit of amubarvimab/romlusevimab was observed for the composite of death or Long COVID (wRR = 0.90, 95% CI 0.64–1.27). The lower bound of the 95% CI of 0.64 suggests that a true risk reduction of Long COVID of 36% or more can reasonably be excluded. There was a significantly lower risk of non-return to health (wRR 0.72) and the composite of non-return to health plus death favored the amubarvimab/romlusevimab arm (wRR 0.65), but with wide 95% CIs (0.52–0.99 and 0.48–0.89) and the caveat that this reflects a significant finding among multiple secondary outcomes evaluated, without adjustment for multiple comparisons.
We found no significant differences between amubarvimab/romlusevimab and placebo arms in proportions of participants reporting any of the 27 symptoms on the long-term diary, including pre-defined symptom categories, frequency of the individual symptoms, or number or severity of symptoms reported. The symptoms reported at week 36, including the most commonly reported ones (fatigue and musculoskeletal pain) were consistent with those reported in other Long COVID studies.24, 25, 26, 27, 28
Additional analyses examining HRQOL measures were consistent with these findings, with no significant benefit of amubarvimab/romlusevimab treatment compared to placebo on these measures at week 36. Using the SF36-v2 instrument, the vast majority of participants reported good, very good, or excellent health (94%) and the same or better health compared to 1 year prior (90%), with similar responses in active and placebo groups. This highlights favorable outcomes in the majority of unvaccinated COVID-19 patients infected with pre-Omicron variants in this study.
Our finding that a highly effective mAb combination demonstrated no meaningful effect on multiple measures of Long COVID at 36 weeks when compared to placebo brings into question the proposed role of viral persistence as a possible pathogenic mechanism in Long COVID,29 as amubarvimab/romlusevimab have long half-lives (44.6–48.6 and 72.2–83.0 days respectively)30 with expected plasma concentrations 100–300-fold in vitro IC90 for the variants in circulation at the time of this trial, and would thus be expected to have ongoing antiviral activity beyond the acute infection period.31, 32, 33 However, this mechanism, and others implicated in the pathogenesis of Long COVID, such as perturbation of inflammatory and coagulation pathways34 were not directly explored in this study. It is also possible that inhibitory concentrations of amubarvimab/romlusevimab were not achieved in the gut, lung, and brain leading to viral persistence in such tissues.35
Limitations of this study include missing diary data for some participants due to attrition/non-response. To address this limitation, inverse probability weighting and multiple supportive analyses were performed to account for the effects of missing data on the primary and secondary outcome measures, with the consistency of effects demonstrating the robustness of our findings. We also acknowledge the potential for survivorship bias in our study, as more deaths occurred in the placebo arm. However, the overall number of deaths within our study was relatively small, limiting the impact of such bias on our findings. We also recognize that the cross-sectional analysis of Long COVID outcomes in this cohort will not capture those persons who may have had symptoms that resolved prior to Week 36 or treatment effects (whether lesser, greater, or no different with respect to Long COVID) prior to week 36. This study was performed before widespread COVID-19 vaccination, which may affect the generalizability of our findings to risk of Long COVID in the current COVID-19 pandemic. Another limitation to the generalizability of our findings is the changing landscape of COVID-19 treatment. The use of anti-SARS-CoV-2 mAbs for COVID-19 treatment is no longer recommended as the current dominant Omicron subvariants in the United States are not expected to be susceptible to these agents. Additionally, it is worth noting that amubarvimab/romlusevimab is no longer in clinical development due to the lack of activity against current circulating variants. We also acknowledge that alternative definitions of Long COVID are in use and that the definition of Long COVID varies widely across studies, making it challenging to compare event rates and findings; however, our investigation incorporating a 27-symptom long-term diary, anchored by informative global assessments, in addition to the use of two additional HRQOL questionnaires and hospitalization/death outcomes, broadly covers commonly accepted measures of Long COVID. The definition of Long COVID used in this analysis is supported by our previous work describing Long COVID in a cohort of participants who had received the mAb bamlanivimab during acute COVID-19 and additional analyses presented in the Supplement for the current analysis population which are consistent with our earlier findings. This definition, assessed in >1200 individuals enrolled from October 2020 to July 2021, distinguishes two participant groups with distinct symptom and return to health profiles following acute COVID-19—we found that all 27 symptoms assessed in the long-term diary were reported with greater frequency among participants meeting our definition of Long COVID than participants who did not. In addition, participants meeting the Long COVID definition were more likely to report not having returned to their usual pre-COVID health than participants who did not.20 In addition, we have explored other outcomes from the long-term diary, such as pre-defined symptom clusters, which represent alternative definitions for Long COVID that have been explored in other published reports. While the EQ-5D-5L and MOS SF-36v2 have not been validated for Long COVID, their history of extensive use in other disease areas and ability to investigate domains now known to be impacted by Long COVID have made them increasingly valuable tools in a growing number of Long COVID studies.36, 37, 38 Finally, we acknowledge that our findings using an effective monoclonal antibody combination may not extend to other effective antivirals or other therapies with different mechanisms of action and pharmacodynamics.9,10
In summary, we report the first prospective study of Long COVID after treatment of acute COVID-19 with an effective monoclonal antibody combination within a large, diverse, randomized, placebo-controlled, blinded clinical trial of outpatients. While amubarvimab/romlusevimab was highly effective in preventing all-cause hospitalizations and deaths in high-risk outpatients with mild-to-moderate COVID-19, there was no meaningful effect of treatment on measures of Long COVID at 36 weeks. Additional interventions are needed for Long COVID prevention.
Contributors
T.H.E, C.M., N.J., D.M.S, M.D.H, and K.W.C conceived and designed the research. Oversight and responsibility for data collection and data verification were delegated by the sponsor to PPD clinical research, a Contract Research Organization (CRO). C.M., J.R., and M.D.H accessed and analyzed the data. T.H.E., C.M., M.D.H, and K.W.C., interpreted the data. T.H.E, and K.W.C drafted the manuscript. All authors reviewed and edited the manuscript.
Data sharing statement
The authors confirm that all data underlying the findings are fully available. Due to ethical restrictions the data are subject to restricted access. Access can be requested by submitting a data request at https://submit.mis.s-3.net/and will require the written agreement of the AIDS Clinical Trials Group (ACTG) and the manufacturer of the investigational product. Requests will be addressed as per ACTG standard operating procedures. Completion of an ACTG Data Use Agreement may be required.
Declaration of interests
Dr. Evering reports other from AIDS Clinical Trials Group (ACTG), during the conduct of the study; personal fees from Tonix Pharmaceuticals, outside the submitted work. Dr. Moser reports grants from NIH/NIAID, grants from NIH/NIAID, during the conduct of the study. Dr. Jilg reports other from AIDS Clinical Trials Group (ACTG), during the conduct of the study; grants from National Institutes of Health (NIH), grants from Harvard University Center for AIDS Research (HU-CFAR), other from Sagent Pharmaceuticals, outside the submitted work. Mr. Ritz reports grants from NIH/NIAID, grants from NIH/NIAID, during the conduct of the study. Dr. Wohl reports grants and personal fees from Gilead Sciences, grants and personal fees from ViiV Healthcare, grants and personal fees from Merck & Co, personal fees from Janssen Pharmaceuticals, personal fees from BMS, personal fees from Theratechnologies, personal fees from EMD Serono, personal fees from Regeneron, outside the submitted work. Dr. Margolis reports other from BRII Biosciences, outside the submitted work. Dr. Eron reports grants from National Institutes of Health, during the conduct of the study; personal fees from Merck & Co, grants and personal fees from Gilead Sciences, other from Invivyd, outside the submitted work. Dr. Currier reports personal fees from Merck, outside the submitted work. Dr. Daar reports grants from NIH, during the conduct of the study; grants and personal fees from Gilead, grants and personal fees from ViiV, personal fees from Theratechnologies, outside the submitted work. Dr. Smith reports grants from NIH, during the conduct of the study; personal fees from Model Medicines, personal fees from Bayer, personal fees from Gilead, personal fees from Lucira, personal fees from VXBiosciences, personal fees from Linear Therapies, personal fees from Red Queen Therapeutics, other from A2 Bio, personal fees from Pharma Holdings, personal fees from Evidera, personal fees from Hyundai, outside the submitted work. Dr. Hughes reports grants from United States National Institutes of Health, during the conduct of the study. Dr. Chew reports grants from NIH/NIAID, grants from NIH/NCATS, during the conduct of the study; personal fees from Pardes Biosciences, outside the submitted work. The remaining authors have no competing interests to disclose.
Acknowledgements
We thank the study participants, site staff, site investigators, and the entire ACTIV-2/A5401 study team; the ACTIV-2 Community Advisory Board; the AIDS Clinical Trials Group, including Lara Hosey, Jhoanna Roa, and Nilam Patel; the Harvard Center for Biostatistics in AIDS Research (CBAR) and ACTG Statistical and Data Analysis Center (SDAC), the National Institute of Allergy and Infectious Diseases (NIAID)/Division of AIDS (DAIDS); the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership; and the PPD clinical research business of Thermo Fisher Scientific.
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI068636, UM1AI068634, and UM1AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Amubarvimab and romlusevimab were supplied by Brii Biosciences.
Footnotes
Trial Registration:ClinicalTrials.gov Identifier: NCT04518410.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102787.
Contributor Information
Teresa H. Evering, Email: evering@med.cornell.edu.
ACTIV-2/A5401 Study Team:
Kara Chew, David (Davey) Smith, Eric Daar, David Wohl, Judith Currier, Joseph Eron, Arzhang Cyrus Javan, Michael Hughes, Carlee Moser, Mark Giganti, Justin Ritz, Lara Hosey, Jhoanna Roa, Nilam Patel, Kelly Colsh, Irene Rwakazina, Justine Beck, Scott Sieg, Jonathan Li, Courtney Fletcher, William Fischer, Teresa H. Evering, Rachel Bender Ignacio, Sandra Cardoso, Katya Corado, Prasanna Jagannathan, Nikolaus Jilg, Alan Perelson, Sandy Pillay, Cynthia Riviere, Upinder Singh, Babafemi Taiwo, Joan Gottesman, Matthew Newell, Susan Pedersen, Joan Dragavon, Cheryl Jennings, Brian Greenfelder, William Murtaugh, Jan Kosmyna, Morgan Gapara, Akbar Shahkolahi, David Margolis, Verónica Lacal, Diego Salusso, Sebastian Nuñez, Marcelo Rodrigo Rodriguez, Luciana Laborde, Marcelo Papasidero, Luis Wehbe, Mariana Gonzalez, Felicitas Fernandez Voena, Tomas Alvarez, Amaru Lopez, Virginia Huhn, Ulises D'Andrea Nores, Pablo Dieser, Fernando Bordese, Marisa Mussi, Rodrigo de Carvalho Santana, Adriana Aparecida Tiraboschi Bárbaro, Breno Santos, Rita de Cássia Alves Lira, Andre Luiz Machado da Silva, Sandra Wagner Cardoso, Maria Pia Diniz Ribeiro, Nathália Soliva, Eduardo Vasconcellos, Jorge Eurico Ribeiro, Miriam Amaral Enéas, Jorge Pinto, Julia Fonseca de Morais Caporali, Flávia Gomes Faleiro Ferreira, Norma Erendira Rivera Martinez, Victor Casildo Bohorquez Lopez, Melchor Victor Frias, Krystle Fetalvero, Alyxzza Maranan, Jennifer Rosa, Thomas Coetzer, Maureen Mohata, Sr., Umesh Lalloo, Sandy Pillay, Penelope Madlala, Larisha Pillay-Ramaya, Jaclyn Ann Bennet, Noluthando Mwelase, Nokuphiwa Mbhele, Frederick Petrick, Leonard Joubert, Rose Mbali, Sr., Natasha Joseph, Mmatsie Manentsa, Eugene van der Walt, Mduduzi Sandile Lawrance Masilela, Zinhle Zwane, Tendai Chiperera, Lerato Mohapi, Suri Moonsamy, Usha Singh, Kirsten McHarry, Elizma Snyman, Pieter Lennox, James Craig Innes, Oteng Letlape, Olebogeng Jonkane, William Brumskine, Tania Adonis, Ni Ni Sein, Modulakgotla Sebe, Yacoob Vahed, Nazreen Jeewa Hussen, Ismail Mitha, Vasundhara Cheekati, Purna Cheekati, Christie Lummus, Samuel Idarraga, Andrew Kim, David N. Pham, Wei-Hsin Kao, Michael M. Pfeffer, Miriam Batule Dominguez, Anju Malik, Anna Bryan, Melanie Arnold, Idania Fernandez, Cinzia Karpf, Aniuska Ruiz, David Taylor, Eric Folkens, Jennifer Manne, Sigal Yawetz, Cheryl Keenan, Emeka Eziri, Carl Fichtenbaum, Jenifer Baer, Sarah Trentman, Robert Call, Leroy Vaughan, Aaron Milstone, Jamie Alex Slandzicki, Jessica Wallan, Clinton Guillory, Nancy Andrews, Leslie Hughes, Teresa H. Evering, Jonathan Berardi, Celine Arar, Randall Quinn, Jorge P. Amaya, Marissa Gomez-Martinez, Luis Cantu, Monica Betancourt-Garcia, Nwora Lance Okeke, Charles M. Burns, Fadi Haddad, Victoria Haddad, Augusto Focil, Griselda Rosas, Susana Moyano, Yaneicy Gonzalez Rojas, Ahmad Aswad, Yevgeniy Bukhman, Manish Jain, Eugene Bukhman, Humam Farah, Rebekah McClain, Eric Daar, Sadia Shaik, Timothy Hatlen, Deepa Gotur, Joseph Surber, Jeffrey Kingsley, April Pixler, Alex Zopo, Jack Herman, Craig Herman, Ramon Leon, Boris Nikolov, Fernando Gonzalez Vergara, Ana I. Gonzalez, Noemi Gonzalez, Michael Gelman, Olga Andriunas, Zarema Jagizarov, Jan Westerman, David Davis, Donna Sherer, Kelly Dooley, Becky Becker, Adaliah Wilkins, Jose Pérez, Eloy Roman, Heriberto Fernández, Bharat Mocherla, Kelly Beck, Valarie Maldonado, Jennifer Veltman, Rajesh Gandhi, Katrina Shea, Matthew Planchon, Laura Bogan Herpel, Kaushlendra K. Tripathi, Donald C. Day, John Pullman, Sr., Erin Williams-Leber, Misty Johnson, Michelle Hecker, Ann Avery, Keila Hoover, George W. Monlux, Elizabeth Juneja, Jr., Arthur Wernick, Karelia Ruiz, Maureen Hernández, Yadilys Pérez, Babafemi O. Taiwo, Claudia Hawkins, Baiba Berzins, Carlos Malvestutto, Heather Harber, Robyn Cicarella, Edwin DeJesus, Charlotte-Paige Rolle, Almena L. Free, Sallie D. Pulliam, Debra Weinstein, Rosa M. Suarez, Ezequiel Socorro, Estefania Socorro, Gene Neytman, Jack Herman, Craig Herman, Raymond Easley, Mariam Aziz, Joan Swiatek, Avish Nagpal, Breanna Kompelien, Kathryn McEvoy, Susan E. Hoover, Allison Lutz, Jessica Just, Manuel Hernandez, Yanly B. Victoria, Gabriel Rodriguez, Upinder Singh, Prasanna Jagannathan, Divya Pathak, Joshua J. Ordway, Megan Heffner, Patrick Weston, Khalilah Weston, Madhu Choudhary, Jennifer Sullivano, Olayemi Osiyemi, Myriam Izquierdo, Odelsey Torna, Brian Clemency, Renoj Varughese, Joshua Lynch, Kara Chew, Aleen Khodabakhshian, Samantha Fortier, Christopher Coyne, Alexandrea Cronin, Constance Benson, Steven Hendrickx, Rosemarie Ramirez, Anne Luetkemeyer, Suzanne Hendler, Dennis Dentoni-Lasofsky, Mobeen Rathore, Saniyyah Mahmoudi, Amna Riaz, Mario Castro, Leslie Spikes, Chase Hall, David Wohl, Jonathan Oakes, Amy James Loftis, Pablo Tebas, William Short, Michael P. Dube, Saahir Khan, Luis M. Mendez, Rachel Bender Ignacio, Sarah McGuffin, Chris Jonsson, Mamta K. Jain, Smruthi Senthil, Kimberly Turner-Gray, Sanjay Mehta, David (Davey) Smith, Mary Lewinski, Masoud Azizad, Christopher Chow, Lisa Nakatani, Derrick Williamson, Hisham Atriss, Matthew Caloura, Midhun Malla, Hannah Hazard-Jenkins, Aimee Wilkin, Jamraus Fayssoux, Hannah Seagle, Rachel Presti, and Alem Haile
Appendix A. Supplementary data
References
- 1.Centers for Disease Control and Prevention Post-COVID conditions: information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html
- 2.Deer R.R., Rock M.A., Vasilevsky N., et al. Characterizing long COVID: deep phenotype of a complex condition. eBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hope A.A., Evering T.H. Postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. Infect Dis Clin North Am. 2022;36(2):379–395. doi: 10.1016/j.idc.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynberg E., Han A.X., Boyd A., et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): a prospective cohort study. Vaccine. 2022;40(32):4424–4431. doi: 10.1016/j.vaccine.2022.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zisis S.N., Durieux J.C., Mouchati C., Perez J.A., McComsey G.A. The protective effect of coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: a multicenter study from a large national health research network. Open Forum Infect Dis. 2022;9(7) doi: 10.1093/ofid/ofac228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y., Choi T., Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med. 2023;183(6):554–564. doi: 10.1001/jamainternmed.2023.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boglione L., Meli G., Poletti F., et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM. 2022;114(12):865–871. doi: 10.1093/qjmed/hcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y., Choi T., Al-Aly Z. Molnupiravir and risk of post-acute sequelae of covid-19: cohort study. BMJ. 2023;381 doi: 10.1136/bmj-2022-074572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramante C.T., Buse J.B., Liebovitz D.M., et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023;23(10):1119–1129. doi: 10.1016/S1473-3099(23)00299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebo K.A., Heath S.L., Fukuta Y., et al. Early antibody treatment, inflammation, and risk of post-COVID conditions. mBio. 2023;14 doi: 10.1128/mbio.00618-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brii Biosciences . 2021. BRII-198 investigator's brochure - edition: 3.0. [Google Scholar]
- 12.Brii Biosciences . BRII-196 investigator’s brochure - edition: 3.0. 2021. [Google Scholar]
- 13.Currier J.S., Moser C., Eron J.J., et al. ACTIV-2: a platform trial for the evaluation of novel therapeutics for the treatment of early COVID-19 in outpatients. J Infect Dis. 2023;228(Suppl 2):S77–S82. doi: 10.1093/infdis/jiad246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evering T.H., Chew K.W., Giganti M.J., et al. Safety and efficacy of combination SARS-CoV-2 neutralizing monoclonal antibodies amubarvimab plus romlusevimab in nonhospitalized patients with COVID-19. Ann Intern Med. 2023;176(5):658–666. doi: 10.7326/M22-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser C.B., Chew K.W., Ritz J., et al. Pooling different placebos as a control group in a randomized platform trial: benefits and challenges from experience in the ACTIV-2 COVID-19 trial. J Infect Dis. 2023;228(Suppl 2):S92–S100. doi: 10.1093/infdis/jiad209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew K.W., Moser C., Daar E.S., et al. Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19. Nat Commun. 2022;13(1):4931. doi: 10.1038/s41467-022-32551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 20.Evering T.H., Moser C.B., Jilg N., et al. Long COVID after bamlanivimab treatment. J Infect Dis. 2023;228(Suppl 2):S126–S135. doi: 10.1093/infdis/jiad286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Hernán M.A., Robins J.M. Chapman & Hall/CRC; Boca Raton: 2020. Causal inference: what if. [Google Scholar]
- 23.Mansournia M.A., Nazemipour M. Recommendations for accurate reporting in medical research statistics. Lancet. 2024;403(10427):611–612. doi: 10.1016/S0140-6736(24)00139-9. [DOI] [PubMed] [Google Scholar]
- 24.Margalit I., Yelin D., Sagi M., et al. Risk factors and multidimensional assessment of long COVID fatigue: a nested case-control study. Clin Infect Dis. 2022;75(10):1688–1697. doi: 10.1093/cid/ciac283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verveen A., Wynberg E., van Willigen H.D.G., et al. Severe fatigue in the first year following SARS-CoV-2 infection: a prospective cohort study. Open Forum Infect Dis. 2022;9(5) doi: 10.1093/ofid/ofac127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteside D.M., Basso M.R., Naini S.M., et al. Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection part 1: cognitive functioning. Clin Neuropsychol. 2022;36:806–828. doi: 10.1080/13854046.2022.2030412. [DOI] [PubMed] [Google Scholar]
- 27.Nolen L.T., Mukerji S.S., Mejia N.I. Post-acute neurological consequences of COVID-19: an unequal burden. Nat Med. 2022;28(1):20–23. doi: 10.1038/s41591-021-01647-5. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Post-COVID conditions: information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html
- 29.Brodin P., Casari G., Townsend L., et al. Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat Med. 2022;28(5):879–882. doi: 10.1038/s41591-022-01766-7. [DOI] [PubMed] [Google Scholar]
- 30.Hao X., Zhang Z., Ma J., et al. Randomized, placebo-controlled, single-blind phase 1 studies of the safety, tolerability, and pharmacokinetics of BRII-196 and BRII-198, SARS-CoV-2 spike-targeting monoclonal antibodies with an extended half-life in healthy adults. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.983505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 32.Certara COVID-19: viral kinetic model 2020. https://www.covidpharmacology.com/in-silico-workbench/viral-kinetic-model/ Available from:
- 33.Hwang I.K., Shih W.J., De Cani J.S. Group sequential designs using a family of type I error probability spending functions. Stat Med. 1990;9(12):1439–1445. doi: 10.1002/sim.4780091207. [DOI] [PubMed] [Google Scholar]
- 34.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: the mount sinai COVID-19 autopsy experience. Mod Pathol. 2021;34(8):1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahmer T., Borzikowsky C., Lieb W., et al. Severity, predictors and clinical correlates of post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. eClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautam N., Madathil S., Tahani N., et al. Medium-term outcomes in severely to critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2022;74(2):301–308. doi: 10.1093/cid/ciab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verveen A., Wynberg E., van Willigen H.D.G., et al. Health-related quality of life among persons with initial mild, moderate, and severe or critical COVID-19 at 1 and 12 months after infection: a prospective cohort study. BMC Med. 2022;20(1):422. doi: 10.1186/s12916-022-02615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.