Summary
Background
In the CALCIPHYX trial, we investigated hexasodium fytate, an inhibitor of vascular calcification, for the treatment of calcific uraemic arteriolopathy (calciphylaxis), a rare condition characterised by painful, non-healing skin lesions.
Methods
In this international, phase 3, randomised, double-blind, placebo-controlled trial, adults with an ulcerated calciphylaxis lesion and pain visual analogue scale (VAS) score ≥50/100 were randomised 1:1 to hexasodium fytate 7 mg/kg or placebo intravenously during maintenance haemodialysis. Primary efficacy outcomes were an 8-item modification of the Bates-Jensen Wound Assessment Tool (BWAT-CUA) and Pain VAS in the intention-to-treat population. ClinicalTrials.gov number: NCT04195906.
Findings
Overall, 34/37 patients randomised to hexasodium fytate and 26/34 patients randomised to placebo completed the 12-week randomised treatment period. At Week 12, both groups (hexasodium fytate versus placebo) showed similar improvements in BWAT-CUA (mean [standard deviation (SD)], −5.3 [5.2] versus −6.0 [6.2]; least squares mean difference, 0.3 [96% confidence interval (CI): −2.5, 3.0]; p = 0.88) and Pain VAS (mean [SD], −19.5 [26.9] versus −32.2 [38.5]; least squares mean difference, 11.5 [96% CI: −4.8, 27.8]; p = 0.15). One patient randomised to placebo briefly received hexasodium fytate in error. Serious adverse events through Week 12 included: calciphylaxis-related events leading to hospitalisation (2/38 [5%] versus 11/33 [33%]) and death (1/38 [3%] versus 5/33 [15%]). During the subsequent 12 weeks of open-label hexasodium fytate and 4 weeks of follow-up, there were no additional calciphylaxis-related events leading to hospitalisation. Over the course of the entire trial, deaths were 2/38 [5%] for the hexasodium fytate group and 7/33 [21%] for the placebo group.
Interpretation
In patients with calciphylaxis, BWAT-CUA and Pain VAS improved similarly in hexasodium fytate- and placebo-treated patients; over the course of the entire trial, there were fewer deaths and calciphylaxis-related events leading to hospitalisation in the hexasodium fytate group.
Funding
Funded by Sanifit, a CSL Vifor company.
Keywords: Calciphylaxis, Calcific uraemic arteriolopathy, Dialysis, SNF472, Hexasodium fytate, Calcification
Research in context.
Evidence before this study
Current clinical practice has not been underpinned by a strong evidence base and has been primarily influenced by case studies and observational studies. Sodium thiosulphate has frequently been used as a treatment for calciphylaxis; however, randomised clinical trials evaluating efficacy have never completed recruitment and results have not been published.
Searches of clinicaltrials.gov, clinicaltrialsregister.eu, pubmed.ncbi.nlm.nih.gov, and scholar.google.com were conducted on 9 July 2024 with the terms “calciphylaxis OR calcific uremic arteriolopathy” and the filter “Phase 3/Phase III.” These searches identified three phase 3 trials that were initiated previously in this area; all studied sodium thiosulphate treatment and all were terminated early due to the inability to accrue subjects.
Hexasodium fytate, a novel agent designed to inhibit vascular calcification by binding to hydroxyapatite, has shown promise in preclinical studies. Phase 1 and 2 trials demonstrated the safety and potential efficacy of hexasodium fytate in reducing hydroxyapatite crystallisation in patients receiving haemodialysis. This provided an evidence base to evaluate the efficacy of hexasodium fytate in the treatment of calciphylaxis.
Added value of this study
The CALCIPHYX trial was the first phase 3, placebo-controlled, randomised trial completed in patients with calciphylaxis. The trial did not meet either of its primary efficacy outcomes. The trial highlights the feasibility of undertaking global, placebo-controlled, randomised clinical trials for this devastating disease which does not have an approved treatment.
Implications of all the available evidence
The findings of the trial prompt further investigation into the mechanisms of action and potential therapeutic benefits of hexasodium fytate, as well as the exploration of novel treatment strategies for calciphylaxis.
Introduction
Calciphylaxis (also referred to as calcific uraemic arteriolopathy or CUA) is a rare but life-threatening condition with an estimated incidence <1% of patients on maintenance dialysis,1, 2, 3, 4, 5 characterised by severely painful, ulcerated skin lesions, predominantly on the trunk or lower limbs.6 The prognosis is poor, with estimated 1-year mortality ranging from approximately 40–70%.5,7,8 There are no approved therapies for calciphylaxis.6 Sodium thiosulphate is often used off-label, based largely on evidence derived from case reports and series.9, 10, 11, 12 A recently published meta-analysis showed no benefit of sodium thiosulphate on survival or healing of skin lesions.13 Treatment goals in calciphylaxis include pain control, prevention of infection (wound and systemic), wound healing and closure, and ultimately, survival.6
It is believed that medial calcification of arterioles in the dermis and subcutaneous adipose tissue in calciphylaxis leads to ischaemia, tissue infarction, and skin necrosis.6 Analysis of skin samples from patients with calciphylaxis has demonstrated the presence of hydroxyapatite in the microvascular and extravascular subcutis, thereby implicating hydroxyapatite crystallisation in the extracellular matrix as a key contributor to disease pathogenesis.14
Myo-inositol hexaphosphate (IP6, phytate) is a naturally occurring substance that binds to hydroxyapatite; IP6 is found in foods with high fibre content but has poor oral bioavailability.15 Hexasodium fytate (also known as SNF472), the hexasodium salt of IP6, was developed as an intravenously administered inhibitor of vascular calcification. Preclinical studies showed that hexasodium fytate binds to hydroxyapatite and prevents the formation and growth of hydroxyapatite crystals ex vivo.16 Phase 1 and phase 2 trials showed that hexasodium fytate had acceptable safety and tolerability. These studies also demonstrated that hexasodium fytate significantly reduces hydroxyapatite crystallisation in patients receiving haemodialysis.17,18 In an open-label, single-arm, phase 2 trial of patients with calciphylaxis, improvements in wound healing, pain, and health-related quality of life were observed in patients treated with hexasodium fytate three times weekly for 12 weeks during each haemodialysis session.19 Herein we describe the primary findings of CALCIPHYX, a randomised, placebo-controlled phase 3 trial of hexasodium fytate in patients with calciphylaxis on maintenance haemodialysis.
Methods
Study design and participants
Protocol details are available online at https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-001301-90/results and https://www.clinicaltrials.gov/study/NCT04195906; a summary of the CALCIPHYX study design was published previously.20 Adult patients on maintenance haemodialysis for at least 2 weeks who had at least one ulcerated calciphylaxis lesion and a pain visual analogue score ≥50/100 provided informed written consent to participate and entered the randomisation. Refer to the protocol for the full list of eligibility criteria. The study included a screening period during which pain medication, wound care, and other background care regimens were stabilised; a 12-week, randomised, double-blind treatment period; a 12-week, open-label treatment period; and a 4-week follow-up period after completion of study treatment (Figure S1).
Ethics
We conducted this international, phase 3, randomised, double-blind, placebo-controlled trial in accordance with the declaration of Helsinki and the International Council for Harmonisation Guidelines on Good Clinical Practice. The study was also conducted in accordance with applicable regional, national, and local regulatory and legal requirements. Before the start of the study, the protocol and other relevant documents were approved by the Institutional Review Board/Independent Ethics Committee for each study centre and relevant regulatory authorities in accordance with local regulatory requirements. Informed consent was obtained from all participants.
Randomisation and masking
Participants were randomly assigned in a 1:1 ratio to receive hexasodium fytate 7 mg/kg or matching placebo. Study drug was administered intravenously over 2.5–3 h during haemodialysis sessions. Patients, investigators, and the analysis team were fully masked to treatment allocation before database lock. To maintain masking, trial medication appearance, packaging, and labelling were identical in both the active treatment and placebo groups, and unique kit codes were used. Off-label use of intravenous sodium thiosulphate was permitted during the study, as were all other usual care treatments except bisphosphonates or intralesional sodium thiosulphate. Randomisation was stratified by use of intravenous sodium thiosulphate.
Outcomes
The study investigator made the clinical diagnosis of calciphylaxis at screening, and at least one calciphylaxis lesion with full thickness ulceration was required for inclusion. A central wound rating group reviewed wound images (photos and videos) collected by sites to confirm that lesions were ulcerated and due to calciphylaxis and to evaluate wound appearance over time.
The central wound rating group assessed wounds quantitatively with the 13-item Bates-Jensen Wound Assessment Tool (BWAT) and qualitatively (improved, worsened, or stayed the same).21 Because the BWAT was originally developed to assess pressure ulcers and there are no validated assessment tools for calciphylaxis-related wounds, we also used an 8-item modification (BWAT-CUA) adapted specifically for this study to attempt to assess prototypical features of calciphylaxis.22
Patients self-reported wound-related pain using a visual analogue scale (VAS). Patients also completed a 17-item Wound–QoL (Quality-of-Life) questionnaire at study visits to rate impairments during the previous 7 days and a daily pain medication diary.
The study had two alternate primary efficacy outcomes, each considered of equal clinical relevance: BWAT-CUA score for the primary lesion and Pain VAS score. The four secondary efficacy outcomes, evaluated hierarchically, were as follows: Wound-QoL score; total BWAT score for the primary lesion; qualitative wound image evaluation for the primary lesion; and opioid use. Safety outcomes included adverse events, serious adverse events, deaths, and calciphylaxis wound-related adverse events (when reporting adverse events, investigators indicated whether they thought an event was calciphylaxis wound-related).
Statistical analysis
The planned enrolment was 66 patients based on a sample size calculation informed by results for wound healing and pain from the phase 2 trial of hexasodium fytate in calciphylaxis.19 The study included a preplanned sample size reassessment when approximately half of the planned number of patients had been treated for 12 weeks.
We used SAS (Cary, NC) Version 9.4 or higher for all analyses. For both alternate primary efficacy outcomes, we compared the absolute change from baseline to Week 12 between treatment groups with a mixed model for repeated measures analysis in the modified intention-to-treat population. The hexasodium fytate intervention was to be considered successful if there were a statistically significant improvement relative to placebo in at least one of these outcomes. Sensitivity analyses for the alternate primary efficacy outcomes included jump-to-placebo and tipping-point approaches to assess the extent to which missing data might have influenced results. To control Type I error inflation, we planned a modified Hochberg procedure with two-sided alpha of 4% for the alternate primary outcomes with 1% alpha retained for testing of secondary outcomes. Further details for planned statistical methods are provided in the Supplementary materials.
Upon review of the prespecified safety analyses, the CALCIPHYX Steering Committee recommended additional post-hoc analyses of death and calciphylaxis-related events. These analyses followed the intention-to-treat principle. We plotted cumulative incidence curves, calculating hazard ratios and 95% confidence intervals (CI). Because of small total numbers of events (all analyses have events totalling ≤15) and early separation of survival curves in each of the time-to-event analyses, the exact Wilcoxon test p-value based on the permutation test was provided. Since the permutation test does not provide an estimated hazard ratio and confidence intervals (CIs), bootstrapping the Cox proportional regression methods was applied to provide the estimated hazard ratio and CIs, adjusting for small numbers of events. Since a sizeable fraction of the population required multiple hospitalisations during the randomised treatment period and there were differences in exposure (higher study retention among patients randomised to hexasodium fytate), we also compared exposure-adjusted event rates between treatment groups, calculating rate ratios and 95% CI using negative binomial regression. Finally, given the extended duration of several hospitalisations, we calculated days alive and out of hospital weighted for exposure and compared treatment groups using the Wilcoxon rank sum test.
Role of the funding source
Authors employed by the funder of the study, CSL Vifor, participated in study design, data collection, data analysis, data interpretation, and writing of the report.
Results
Patient population
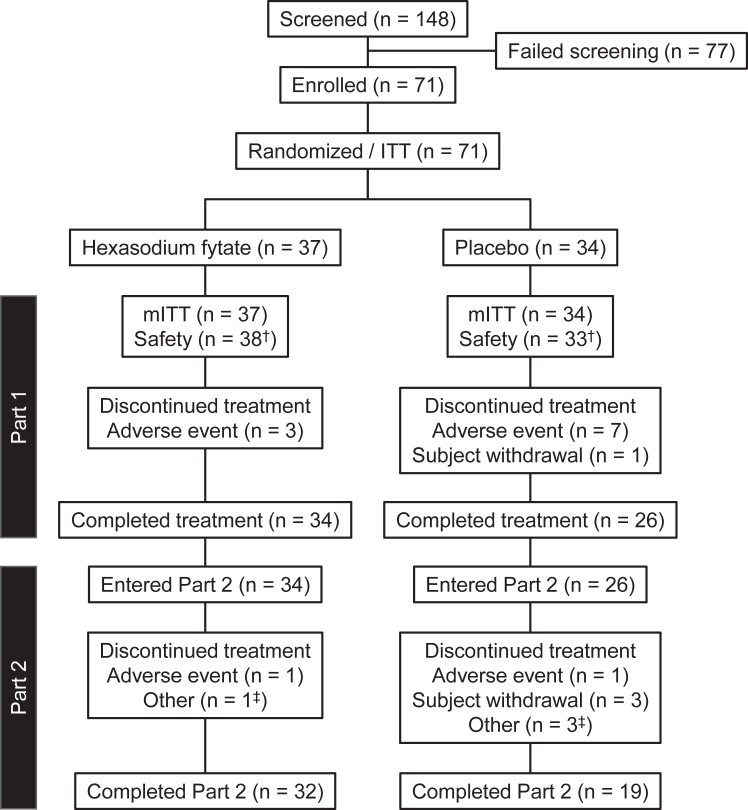
Between February 2020 and April 2022, we screened 148 patients at 74 centres, of whom 77 did not meet study eligibility criteria. The central wound rating group primarily excluded patients due to the presence of potential alternative diagnoses that could explain the ulcers (Table S2). We randomly assigned 71 patients to hexasodium fytate (n = 37) or placebo (n = 34). All randomised patients were included in the modified intention-to-treat population for efficacy and the safety population. One patient in the placebo group, who inadvertently received double-blind hexasodium fytate during Weeks 11 and 12, was analysed for efficacy in the placebo group and for safety in the hexasodium fytate group (Fig. 1).
Fig. 1.
Enrolment and disposition of study participants. ITT, intention-to-treat analysis set; mITT, modified intention-to-treat analysis set. †One subject randomised to the placebo group received hexasodium fytate during Week 11 and Week 12 in Part 1. The subject was included in the placebo group of the ITT and mITT populations for efficacy analyses and in the hexasodium fytate group of the Safety Analysis Population for safety analyses. ‡Other reasons for discontinuation of treatment in Part 2: hexasodium fytate, expired study drug (1 patient); placebo, unable to administer study drug, ampule broken, and scheduling issues (1 patient each).
Baseline demographic and clinical characteristics of the patients were similar in both groups (Table 1). Mean (standard deviation [SD]) age was 57.5 (11.7) years and 44 patients (62%) were women. At baseline, intravenous sodium thiosulphate was administered to 49 patients (69%) and opioids were administered to 50 patients (70%); these medications could be continued during the study. A higher proportion of patients randomised to hexasodium fytate completed the 12-week randomised treatment period (34/37 [92%] versus 26/34 [76%]).
Table 1.
Baseline demographic and clinical characteristics.
| Hexasodium fytate (n = 37) | Placebo (n = 34) | Total (n = 71) | |
|---|---|---|---|
| Mean age, SD | 57.7 (12.1) | 57.2 (11.3) | 57.5 (11.7) |
| Female sex | 23 (62%) | 21 (62%) | 44 (62%) |
| Race | |||
| White | 20 (54%) | 18 (53%) | 38 (54%) |
| Black or African American | 14 (38%) | 12 (35%) | 26 (37%) |
| Other | 3 (8%) | 4 (12%) | 7 (10%) |
| Region | |||
| North America | 32 (86%) | 32 (94%) | 64 (90%) |
| Europe | 5 (14%) | 2 (6%) | 7 (10%) |
| Mean BMI (kg/m2), SD | 33.2 (9.3) | 34.3 (9.0) | 33.7 (9.2) |
| Mean time on haemodialysis (years), SD | 5.0 (5.1) | 4.6 (4.7) | 4.8 (4.9) |
| Mean time since ESKD diagnosis (years), SD | 5.7 (4.9) | 4.9 (4.5) | 5.3 (4.7) |
| Use of other medications at baselinea | |||
| Sodium thiosulphate | 26 (68%) | 23 (70%) | 49 (69%) |
| Calcimimetics | 20 (53%) | 16 (48%) | 36 (51%) |
| Non–calcium-based phosphate binder | 29 (76%) | 26 (79%) | 55 (78%) |
| Calcium-based phosphate binder | 8 (21%) | 8 (24%) | 16 (23%) |
| Vitamin D compound | 16 (42%) | 20 (61%) | 36 (51%) |
| Warfarin | 4 (11%) | 4 (12%) | 8 (11%) |
| Opioids | 25 (66%) | 25 (76%) | 50 (70%) |
| Mean baseline BWAT-CUA scoreb (8–40), SD | 18.9 (5.3) | 20.8 (5.0) | 19.8 (5.2) |
| Mean baseline Pain VAS score (0–100), SD | 67.0 (27.0) | 71.3 (29.0) | 69.1 (27.9) |
BMI, body mass index; BWAT, Bates-Jensen Wound Assessment Tool; CUA, calcific uraemic arteriolopathy; ESKD, end-stage kidney disease; SD, standard deviation; VAS, visual analogue scale.
Data are presented as n (%).
Percentages for baseline medication use were calculated for the safety analysis population (n = 38 hexasodium fytate; n = 33 placebo).
Baseline values for each component of the BWAT are provided in the Supplementary materials.
Alternate primary efficacy outcomes
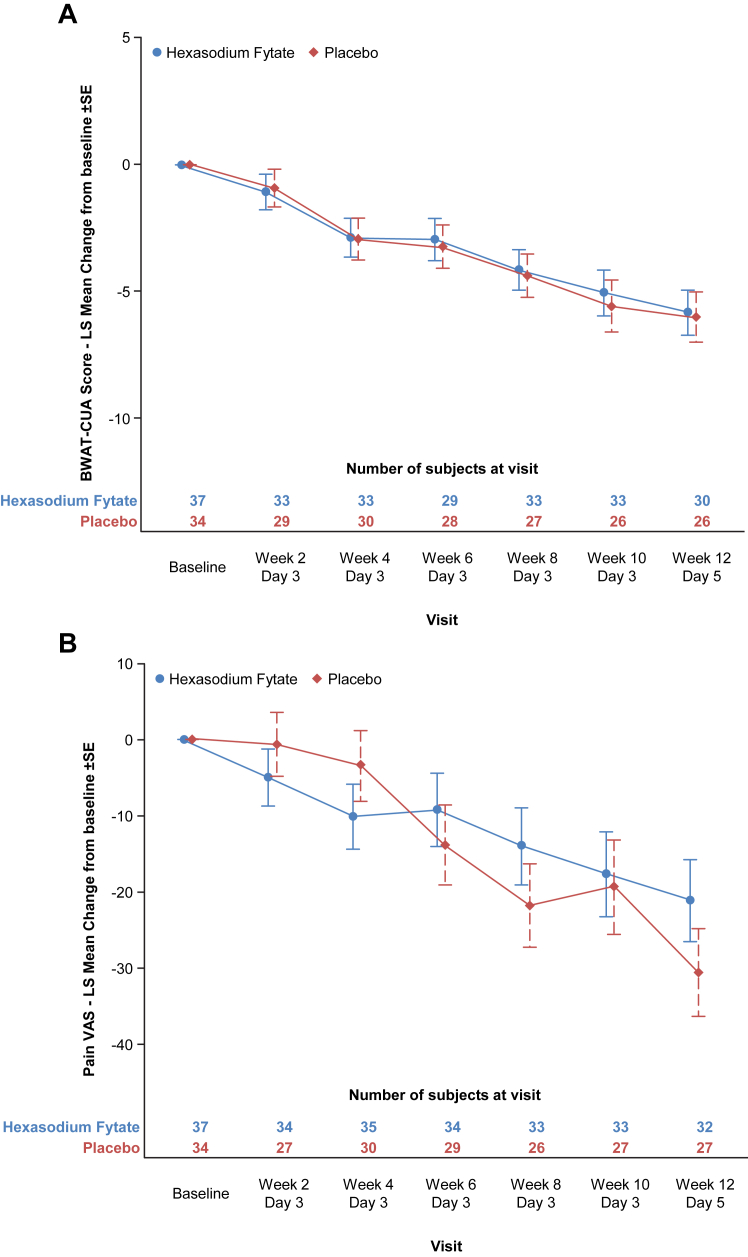
For the alternate primary efficacy outcomes (Fig. 2), BWAT-CUA and Pain VAS scores improved from baseline to Week 12 in both treatment groups. Mean (SD) changes for BWAT-CUA were −5.3 (5.2) in the hexasodium fytate group and −6.0 (6.2) in the placebo group, corresponding to least squares mean (±SE) difference of 0.3 ± 1.3 (96% CI, −2.5 to 3.0; p = 0.88). Mean (SD) changes for Pain VAS were −19.5 (26.9) in the hexasodium fytate group and −32.2 (38.5) in the placebo group, corresponding to least squares mean (±SE) difference of 11.5 ± 7.9) (96% CI, −4.8 to 27.8; p = 0.15). Sensitivity analyses did not yield materially different results.
Fig. 2.
Primary efficacy outcomes. BWAT, Bates-Jensen Wound Assessment Tool; CUA, calcific uraemic arteriolopathy (calciphylaxis); LS, least squares; SE, standard error and VAS, visual analogue scale. ∗Shown are results in the modified intention-to-treat population for alternate primary outcomes of mean ± SE change from baseline to Week 12 in BWAT-CUA score (Panel A) and mean ± SE change from baseline to Week 12 in Pain VAS Score (Panel B).
Secondary efficacy outcomes
The least squares mean difference in Wound-QoL from baseline to week 12 (hexasodium fytate versus placebo) was 0.1 (96% CI, −0.4 to 0.6; p = 0.71) and for BWAT total score −0.0 (96% CI, −5.3 to 5.2; p = 0.99) (Table S3). For qualitative wound image evaluation of the primary lesion at Week 12, the odds of relative improvement (hexasodium fytate versus placebo) were 1.5 (95% CI, 0.6–4.1; p = 0.38). For rate of change in daily opioid use, the corresponding difference in slope estimates was 0.6 (95% CI, −0.8 to 1.9; p = 0.41).
Safety outcomes
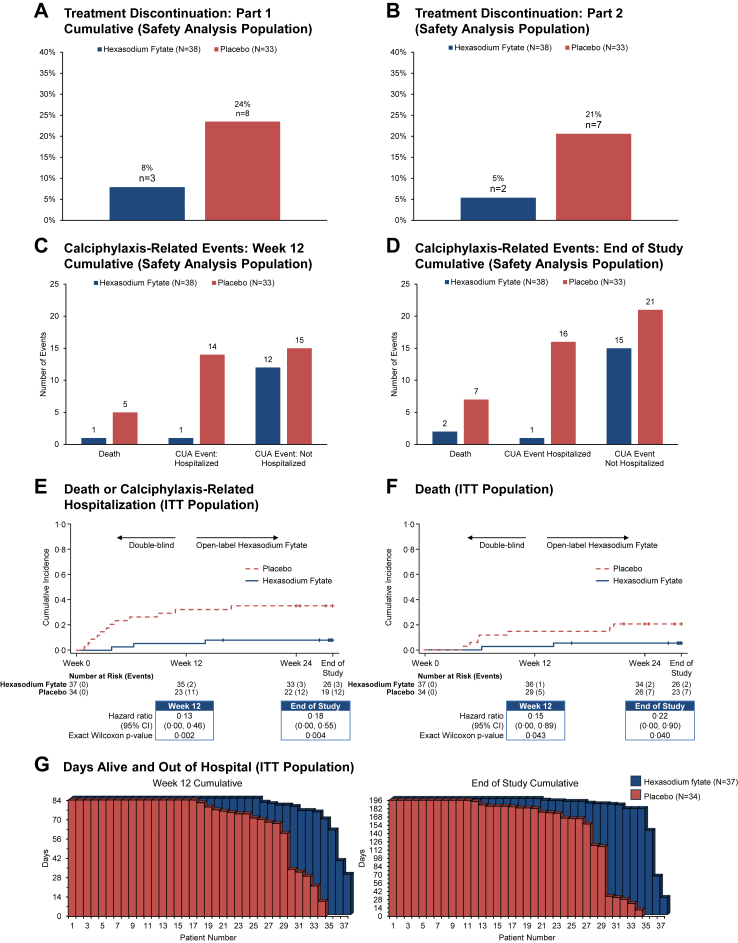
Incidences of treatment-emergent adverse events in each part of the study are summarised in Table 2. Treatment emergent adverse events led to treatment discontinuation for fewer patients in the hexasodium fytate group (2/38 [5%]) than the placebo group (6/33 [18%]). The proportion of patients during the double-blind period with calciphylaxis-related infections (1/38 [3%] versus 7/33 [21%]), calciphylaxis-related events leading to hospitalisation (2/38 [5%] versus 11/33 [33%]) and death (1/38 [3%] versus 5/33 [15%]) were lower in patients randomised to hexasodium fytate versus placebo (Fig. 3, panels A–D). During the 12-week open-label and 4-week follow-up periods, there were no further calciphylaxis-related events leading to hospitalisation.
Table 2.
Safety outcomes.
| Outcome | Part 1: double-blind |
Part 2: open-label |
Follow-up |
|
|---|---|---|---|---|
| Hexasodium fytate (n = 38) | Placebo (n = 33) | Hexasodium fytate (n = 60) | Off treatment (n = 71a) | |
| Any TEAE | 33 (87%) | 31 (94%) | 48 (80%) | 20 (28%) |
| Calciphylaxisb | 10 (26%) | 11 (33%) | 4 (7%) | 0 (0%) |
| Cellulitis | 3 (8%) | 4 (12%) | 1 (2%) | 2 (3%) |
| Pain in extremity | 4 (11%) | 3 (9%) | 5 (8%) | 2 (3%) |
| Headache | 3 (8%) | 3 (9%) | 0 (0%) | 1 (1%) |
| Nausea | 2 (5%) | 4 (12%) | 3 (5%) | 0 (0%) |
| Vomiting | 5 (13%) | 1 (3.0%) | 2 (3%) | 0 (0%) |
| Arthralgia | 5 (13%) | 0 (0%) | 1 (2%) | 1 (1%) |
| Fall | 3 (8%) | 2 (6%) | 5 (8%) | 0 (0%) |
| Hypervolemia | 4 (11%) | 0 (0%) | 2 (3%) | 0 (0%) |
| Serious TEAEs | 13 (34%) | 17 (52%) | 18 (30%) | 6 (8%) |
| TEAE related to study drug | 0 (0%) | 1 (3%) | 5 (8%) | 0 (0%) |
| TEAE leading to treatment discontinuation | 2 (5%) | 6 (18%) | 2 (3%) | 1 (1%)c |
| TEAE leading to death | 1 (3%) | 6 (18%) | 1 (2%) | 1 (1%) |
| Calciphylaxis wound-related TEAE | 11 (29%) | 16 (49%) | 3 (5%) | 1 (1%) |
| Calciphylaxis-related infection/infestation | 1 (3%) | 7 (21%) | 1 (2%) | 0 (0%) |
TEAE, treatment-emergent adverse event that was new or worsened in that part of the study.
Preferred terms are shown for TEAEs reported in >2 patients in either group during double-blind treatment.
Data are presented as n (%).
Includes all 71 randomised patients from Part 1, regardless of whether they entered Part 2.
Worsening of calciphylaxis after randomisation was reported as a TEAE.
One patient received last dose of study drug in the 12-week randomisation period and did not complete Part 1 due to a serious TEAE. The patient withdrew from the study due to a TEAE leading to death in the follow-up period.
Fig. 3.
Treatment discontinuation, death, calciphylaxis-related events, and hospitalisation. CI, confidence interval; CUA, calcific uraemic arteriolopathy (calciphylaxis); ITT, intention-to-treat. Panels A and B show treatment discontinuation rates by treatment group in the Safety Analysis Population for Part 1 (A) and Part 2 (B). Panels C and D show the incidences of death, calciphylaxis-related events (for each adverse event, the investigator reported whether the event was related to calciphylaxis) leading to hospitalisation, and calciphylaxis-related events not leading to hospitalisation in the Safety Analysis Population through Week 12 cumulative (C) and through end of study (D). Panels E and F show Kaplan–Meier analyses of the composite of death or calciphylaxis-related hospitalisation (E) and death (F) in the ITT population. Panel G shows days alive and out of hospital weighted for exposure, by patient, through Week 12 and through end of study in the ITT Population.
Post-hoc efficacy endpoints
The hazard ratio for the composite of death or calciphylaxis-related hospitalisation was 0.13 (95% CI, 0.00–0.46; nominal p = 0.002) at Week 12 and 0.18 (95% CI, 0.00–0.55; nominal p = 0.004) at end of study. The hazard ratio for death was 0.15 (95% CI, 0.00–0.89; nominal p = 0.043) at Week 12 and 0.22 (95% CI, 0.00–0.90; nominal p = 0.040) at end of study. During the randomised treatment period, the total number of deaths and calciphylaxis-related hospitalisations was 2 in the hexasodium fytate group and 19 in the placebo group, yielding exposure-adjusted event rates of 0.018 and 0.248, corresponding to a rate ratio of 0.074 (95% CI, 0.022–0.253; nominal p < 0.001). The total number of deaths and calciphylaxis-related events (including non-hospitalised events) was 14 in the hexasodium fytate group and 35 in the placebo group, yielding exposure-adjusted event rates of 0.111 and 0.371, corresponding to a rate ratio of 0.298 (95% CI, 0.133–0.668; nominal p = 0.003). From randomisation until end of study, the cumulative number of death or calciphylaxis-related hospitalisations was 3 in the hexasodium fytate group and 23 in the placebo group, yielding exposure-adjusted event rates of 0.013 and 0.204, respectively, corresponding to a rate ratio of 0.063 (95% CI, 0.018–0.217; nominal p < 0.001). From randomisation until end of study, the cumulative number of death or calciphylaxis-related events (including non-hospitalised events) was 18 in the hexasodium fytate group and 44 in the placebo group, yielding exposure-adjusted event rates of 0.061 and 0.263 respectively, corresponding to a rate ratio of 0.231 (95% CI, 0.096–0.554; nominal p = 0.001). Mean (SD) days alive and out of hospital were 78.9 (12.0) and 71.4 (20.6) in the hexasodium fytate and placebo groups, respectively, during the randomised treatment period (nominal p = 0.07), and 182.8 (34.6) and 156.7 (58.8), respectively, until the conclusion of the study (Wilcoxon-Mann-Whitney test p = 0.007) (Fig. 3, panels E–G).
Discussion
This randomised, placebo-controlled trial of hexasodium fytate in patients receiving maintenance haemodialysis with calciphylaxis was designed to compare BWAT-CUA and/or Pain VAS scores and did not yield improvements in its alternate primary efficacy outcomes, as patients in both groups experienced improvements in both parameters. No significant differences were observed in prespecified secondary efficacy outcomes, including the Wound-QoL questionnaire, total BWAT score, a qualitative assessment of wound healing, and use of narcotic analgesics.
Hexasodium fytate was well tolerated compared with placebo. Treatment-emergent adverse events were typical of the patient population. Patients randomised to hexasodium fytate were more likely to remain on study drug. The proportion of patients who died or who experienced calciphylaxis-related events, including infection and hospitalisation, was numerically lower in the hexasodium fytate group. In the post-hoc analysis of composite endpoints of time to death or first calciphylaxis-related hospitalisation, time to death or any hospitalisation, or days alive and out of hospital, patients randomised to hexasodium fytate showed clinically meaningful improvements that were of nominal statistical significance. Patients with calciphylaxis are at increased risk of bacteraemia,7 which is usually managed by hospitalisation. Post hoc analysis indicated patients treated with SNF472 had fewer calciphylaxis wound-related hospitalisations. The primary outcome measures of BWAT-CUA and Pain VAS had not been previously validated as endpoints in calciphylaxis. Due to the medical complexity of the patient population, these endpoints may not be sensitive enough to detect potential improvements specific to the calciphylaxis wounds and the impact on important clinical outcomes such as calciphylaxis-associated hospitalisation and death. This may also be influenced by the pre-specified methodology to handle missing data; the increased number of deaths and calciphylaxis hospitalisations seen in the placebo group led to a substantially larger amount of missing data in the placebo group. The study duration may also not have been long enough to see a difference in the primary endpoints. The improvement in BWAT-CUA and Pain VAS may reflect better than usual standard of care of the wound and pain management in the context of a clinical trial.
Favourable effects of hexasodium fytate on vascular calcification are plausible based on prior experience. In a randomised, placebo-controlled trial of 274 patients receiving maintenance haemodialysis with coronary artery calcification at baseline (but without calciphylaxis), hexasodium fytate significantly attenuated the progression of coronary artery and aortic valve calcification relative to placebo and was safe and well tolerated.23 The role of vascular calcification in calciphylaxis has been described for more than 60 years,24 but the precise pathogenesis continues to be investigated and elucidated.6 In patients undergoing haemodialysis, progressive calcification of skin arterioles, typically in areas with abundant adipose tissue such as the abdomen and thighs, can result in subintimal fibrosis and thrombosis, arteriolar occlusion, ischaemia, and progressive necrosis, which cause the painful skin ulcerations of calciphylaxis.6 The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial investigated the effects of cinacalcet on cardiovascular outcomes associated with vascular calcification in patients receiving maintenance haemodialysis with secondary hyperparathyroidism.25 A multivariable analysis of EVOLVE safety data showed that randomisation to cinacalcet resulted in a 75% lower risk of calciphylaxis (hazard ratio, 0.25; 95% CI, 0.10–0.67), suggesting that the risk of calciphylaxis may be reduced when vascular calcification is attenuated.26 Cinacalcet has not been evaluated as a treatment for calciphylaxis.
Hexasodium fytate, an intravenously administered salt of naturally occurring IP6, was specifically developed to treat dystrophic calcification including calciphylaxis by binding to hydroxyapatite with high affinity, thereby preventing formation and growth of the hydroxyapatite crystals responsible for medial calcification.16 Hexasodium fytate does not chelate free calcium, supporting its specificity to modulate vascular calcification.16 Binding of hexasodium fytate to hydroxyapatite occurs rapidly (within minutes) and persists for days, which allows for administration of hexasodium fytate 3 times weekly during haemodialysis.16 Many other treatments are commonly administered empirically to treat calciphylaxis, including bisphosphonates, sodium thiosulphate, vitamin K, phosphate binders, and calcimimetics, but none target hydroxyapatite formation and growth, and none have been shown to improve outcomes compared with placebo in a clinical trial. Before this study, available data on calciphylaxis and its treatment were limited to observational and single-arm interventional studies, and as a result, treatment strategies for calciphylaxis are unproven and vary widely.4,5,7
Given the relatively low prevalence of calciphylaxis, we did not believe that an event-driven trial would be feasible due to anticipated challenges in recruitment. Thus, we needed to design a trial of modest size to test whether hexasodium fytate could improve clinically relevant features of calciphylaxis. We settled on a quantitative assessment of the primary calciphylaxis lesion and a patient-reported assessment of pain as alternate primary outcomes. The BWAT was developed initially as a tool for assessment of pressure ulcers; we developed the BWAT-CUA (a subset of questions from the BWAT) for this trial in an effort to adapt it for calciphylaxis wounds.22 While the BWAT-CUA has face validity, and appeared to be sensitive to change when applied to Phase 2 study results, it had not been prospectively tested before its use here. With respect to the Pain VAS, pain is perceived differently by different patients, and the provision of, and response to, narcotic analgesic and other analgesic agents and agents aimed to manage different types of pain may vary, losing specificity.27 Validated markers of disease progression and/or pragmatic trial design28 could facilitate future clinical trials in calciphylaxis.
CALCIPHYX was the first phase 3, randomised, controlled trial to achieve recruitment targets and complete double-blinded treatment in patients with calciphylaxis. Patients were enrolled and treated at sites across the United States and Europe, with close collaboration among study investigators and large dialysis organisations. These achievements are notable not only because the trial was conducted successfully during the global COVID-19 pandemic, but also because three randomised clinical trials of sodium thiosulphate for calciphylaxis were previously attempted; all were terminated during patient recruitment (NCT03150420, NCT02527213, ISRCTN73380053), the most recent of which recruited 29 patients over three years before the pandemic. Given the frequent, empirical use of sodium thiosulphate to manage calciphylaxis, randomisation was stratified by sodium thiosulphate use. Results for primary efficacy outcomes were similar among patients treated and not treated with sodium thiosulphate.
The major limitation of CALCIPHYX was the small sample size related primarily to its application in a rare disease. Thinking that an event-driven trial would be infeasible, we employed two patient-centred alternate primary efficacy outcomes, neither of which had been prospectively validated in calciphylaxis. This trial was conducted in patients receiving maintenance hemodialysis. Further studies are warranted in other susceptible patient groups including those receiving peritoneal dialysis and those with advanced, non-dialysis-requiring chronic kidney disease.
In sum, among patients receiving maintenance haemodialysis with calciphylaxis, a 12-week intervention with hexasodium fytate did not yield improvements in the alternate primary efficacy outcomes—a quantitative, unvalidated, assessment of the primary calciphylaxis lesion and a patient-reported visual analogue scale for pain—and did not achieve its prespecified secondary efficacy outcomes. However, results of the safety analysis showed numerically lower rates of death and calciphylaxis-related events in patients treated with hexasodium fytate, which were further supported by post-hoc, hypothesis-generating, intention-to-treat analyses of composite endpoints, including days alive and out of hospital.
Contributors
SS, SUN, JP, AG, and GMC conceptualised the study.
GA, LHK, JP, AG, and KJC did the formal analysis.
JP and AG acquired funding.
SS, SUN, VB, LJG, TES, SMM, GRA, DKC, JLH, and GMC did the investigation.
SS, SUN, KJC, JP, AG, and GMC did the methodology.
GA and LHK did the project administration.
GA, LHK, JP, and AG supervised the study.
SS, SUN, KJC, GA, LHK, JP, AG, and GMC did the data visualisation.
SS, KJC, LHK, JP, and GMC wrote the original draft.
KJC, GA, and LHK accessed and verified the raw data.
All authors had access to all the data and had final responsibility for the decision to submit for publication. All authors curated and verified the data, were responsible for resources, and reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
CSL will consider on a case-by-case basis requests to share Individual Patient Data (IPD) from CSL sponsored studies with external bona-fide, qualified scientific and medical researchers. When appropriate, IPD will generally be shared once review by major regulatory authorities (ie, FDA, EMA) is complete and the primary publication is available. Proposed research should seek to answer a previously unanswered important medical or scientific question. Requests should reflect those important questions. Applicable country specific privacy and other laws and regulations will be considered and may prevent sharing of IPD.
A research proposal detailing the use of the IPD will be reviewed by an internal CSL review committee. If the request is approved, and the researcher agrees to the applicable terms and conditions in a data sharing agreement, IPD that has been appropriately anonymized will be made available. Supporting documents including study protocol and Statistical Analysis Plan will also be provided to the researcher.
For information on the process and requirements for submitting a voluntary data sharing request for IPD, please contact CSL at clinicaltrials@cslbehring.com.
Declaration of interests
SS received consulting fees from Sanifit and Inozyme Pharma Inc; honoraria from AstraZeneca, Bayer, Sanofi-Genzyme, Novartis, CSL Vifor, GSK, Menarini, Medscape, and Boehringer-Ingelheim; support for attending meetings and/or travel from Sanifit, AstraZeneca, Novartis, and CSL Vifor. SUN received research funding to institution from Laboratoris Sanifit, CSL/Vifor, Hope Pharma, Inozyme Pharma, Epizon; consulting fees from Laboratoris Sanifit, CSL/Vifor, Inozyme Pharma, and Epizon; medical writing support from Laboratoris Sanifit and CSL/Vifor; royalties from UpToDate; and support for attending meetings and/or travel from Inozyme Pharma. LJG received consulting fees from Sanifit Therapeutics. SMM received consulting fees from Ardelyx, Sanfit, and Inozyme; and holds stock or stock options in Eli Lilly. DKC is an employee of Fresenius Medical Care and declares spousal stock in Amgen. KJC received consulting fees from Sanifit srl and Vifor Pharma. GA is an employee of CSL Vifor. LHK was an employee of and received stock compensation from CSL Vifor during the study. JP received consulting fees and stocks or stock options from Sanifit Therapeutics, which owns or has rights on patents related to this work where JP is a coinventor. AG was an employee of Sanifit Therapeutics and CSL Vifor and received consulting fees from Sanifit Therapeutics and CSL Vifor. GMC received graphics support (no writing support) from Sanifit, Vifor, and CSL Behring; received consulting fees from Sanifit, Akebia, AstraZeneca, CSL Behring, and Vertex; participated on a data safety monitoring board or Advisory Board for Ardelyx, Calico, and Miromatrix; served in a leadership or fiduciary role for Satellite Healthcare; received stock or stock options from CloudCath, Duract, Eliaz Therapeutics Outset, Renibus, and Unicycive. All other authors declare no competing interests.
Acknowledgements
We thank all study participants and investigators. Primary funding for this study was provided by Sanifit, a CSL Vifor company. Jonathan Latham provided medical writing support, with funding from Sanifit.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102784.
Appendix A. Supplementary data
References
- 1.Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498–503. doi: 10.1007/s10157-013-0782-z. [DOI] [PubMed] [Google Scholar]
- 2.Nigwekar S.U., Solid C.A., Ankers E., et al. Quantifying a rare disease in administrative data: the example of calciphylaxis. J Gen Intern Med. 2014;29(Suppl 3):S724–S731. doi: 10.1007/s11606-014-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigwekar S.U., Zhao S., Wenger J., et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421–3429. doi: 10.1681/ASN.2015091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandenburg V.M., Kramann R., Rothe H., et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32:126–132. doi: 10.1093/ndt/gfv438. [DOI] [PubMed] [Google Scholar]
- 5.Ruderman I., Toussaint N.D., Hawley C.M., et al. The Australian Calciphylaxis Registry: reporting clinical features and outcomes of patients with calciphylaxis. Nephrol Dial Transplant. 2021;36:649–656. doi: 10.1093/ndt/gfz256. [DOI] [PubMed] [Google Scholar]
- 6.Nigwekar S.U., Thadhani R., Brandenburg V.M. Calciphylaxis. N Engl J Med. 2018;378:1704–1714. doi: 10.1056/NEJMra1505292. [DOI] [PubMed] [Google Scholar]
- 7.Chinnadurai R., Huckle A., Hegarty J., Kalra P.A., Sinha S. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis study. J Nephrol. 2021;34:1537–1545. doi: 10.1007/s40620-020-00908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weenig R.H., Sewell L.D., Davis M.D., McCarthy J.T., Pittelkow M.R. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569–579. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 9.Peng T., Zhuo L., Wang Y., et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton) 2018;23:669–675. doi: 10.1111/nep.13081. [DOI] [PubMed] [Google Scholar]
- 10.Udomkarnjananun S., Kongnatthasate K., Praditpornsilpa K., Eiam-Ong S., Jaber B.L., Susantitaphong P. Treatment of calciphylaxis in CKD: a systematic review and meta-analysis. Kidney Int Rep. 2019;4:231–244. doi: 10.1016/j.ekir.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigwekar S.U., Brunelli S.M., Meade D., Wang W., Hymes J., Lacson E., Jr. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162–1170. doi: 10.2215/CJN.09880912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlieper G., Brandenburg V., Ketteler M., Floege J. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5:539–543. doi: 10.1038/nrneph.2009.99. [DOI] [PubMed] [Google Scholar]
- 13.Wen W., Portales-Castillo I., Seethapathy R., et al. Intravenous sodium thiosulphate for calciphylaxis of chronic kidney disease: a systematic review and meta-analysis. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramann R., Brandenburg V.M., Schurgers L.J., et al. Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant. 2013;28:856–868. doi: 10.1093/ndt/gfs466. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S., Raggi P., Chertow G.M. SNF472: mechanism of action and results from clinical trials. Curr Opin Nephrol Hypertens. 2021;30:424–429. doi: 10.1097/MNH.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 16.Perelló J., Ferrer M.D., Perez MdM., et al. Mechanism of action of SNF472, a novel calcification inhibitor to treat vascular calcification and calciphylaxis. Br J Pharmacol. 2020;177:4400–4415. doi: 10.1111/bph.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perelló J., Joubert P.H., Ferrer M.D., Canals A.Z., Sinha S., Salcedo C. First-time-in-human randomized clinical trial in healthy volunteers and haemodialysis patients with SNF472, a novel inhibitor of vascular calcification. Br J Clin Pharmacol. 2018;84:2867–2876. doi: 10.1111/bcp.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salcedo C., Joubert P.H., Ferrer M.D., et al. A phase 1b randomized, placebo-controlled clinical trial with SNF472 in haemodialysis patients. Br J Clin Pharmacol. 2019;85:796–806. doi: 10.1111/bcp.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandenburg V.M., Sinha S., Torregrosa J.V., et al. Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: a phase 2 open-label study of patients with calciphylaxis. J Nephrol. 2019;32:811–821. doi: 10.1007/s40620-019-00631-0. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S., Gould L.J., Nigwekar S.U., et al. The CALCIPHYX study: a randomized, double-blind, placebo-controlled, Phase 3 clinical trial of SNF472 for the treatment of calciphylaxis. Clin Kidney J. 2022;15:136–144. doi: 10.1093/ckj/sfab117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates-Jensen B.M., Vredevoe D.L., Brecht M.L. Validity and reliability of the pressure sore status tool. Decubitus. 1992;5:20–28. [PubMed] [Google Scholar]
- 22.Gould L.J., Serena T.E., Sinha S. Development of the BWAT-CUA scale to assess wounds in patients with calciphylaxis. Diagnostics. 2021;11:730. doi: 10.3390/diagnostics11040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raggi P., Bellasi A., Bushinsky D., et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized phase 2b study. Circulation. 2020;141:728–739. doi: 10.1161/CIRCULATIONAHA.119.044195. [DOI] [PubMed] [Google Scholar]
- 24.Selye H. University of Chicago Press; Chicago, IL: 1962. Calciphylaxis. [Google Scholar]
- 25.Chertow G.M., Block G.A., Correa-Rotter R., et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 26.Floege J., Kubo Y., Floege A., Chertow G.M., Parfrey P.S. The effect of cinacalcet on calcific uremic arteriolopathy events in patients receiving hemodialysis: the EVOLVE trial. Clin J Am Soc Nephrol. 2015;10:800–807. doi: 10.2215/CJN.10221014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed M.D., Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol. 2014;54:241–244. doi: 10.1002/jcph.250. [DOI] [PubMed] [Google Scholar]
- 28.Meinecke A.K., Welsing P., Kafatos G., et al. Series: pragmatic trials and real world evidence: paper 8. Data collection and management. J Clin Epidemiol. 2017;91:13–22. doi: 10.1016/j.jclinepi.2017.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.