Abstract
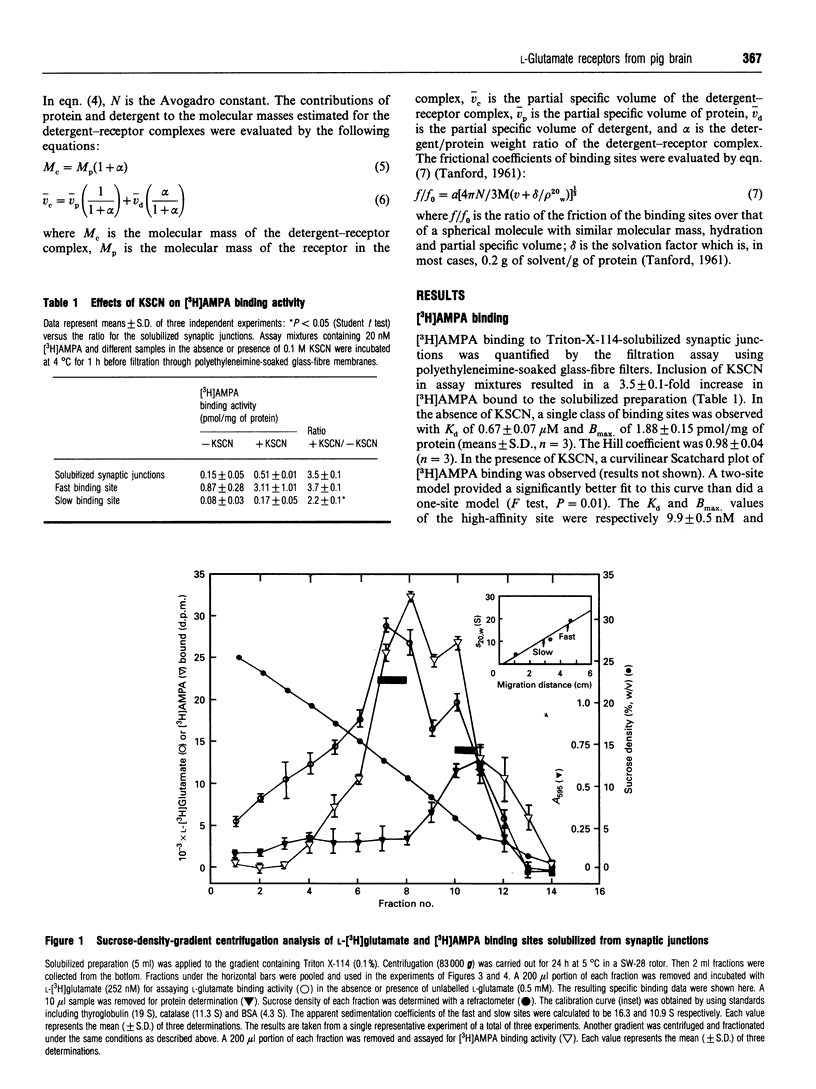
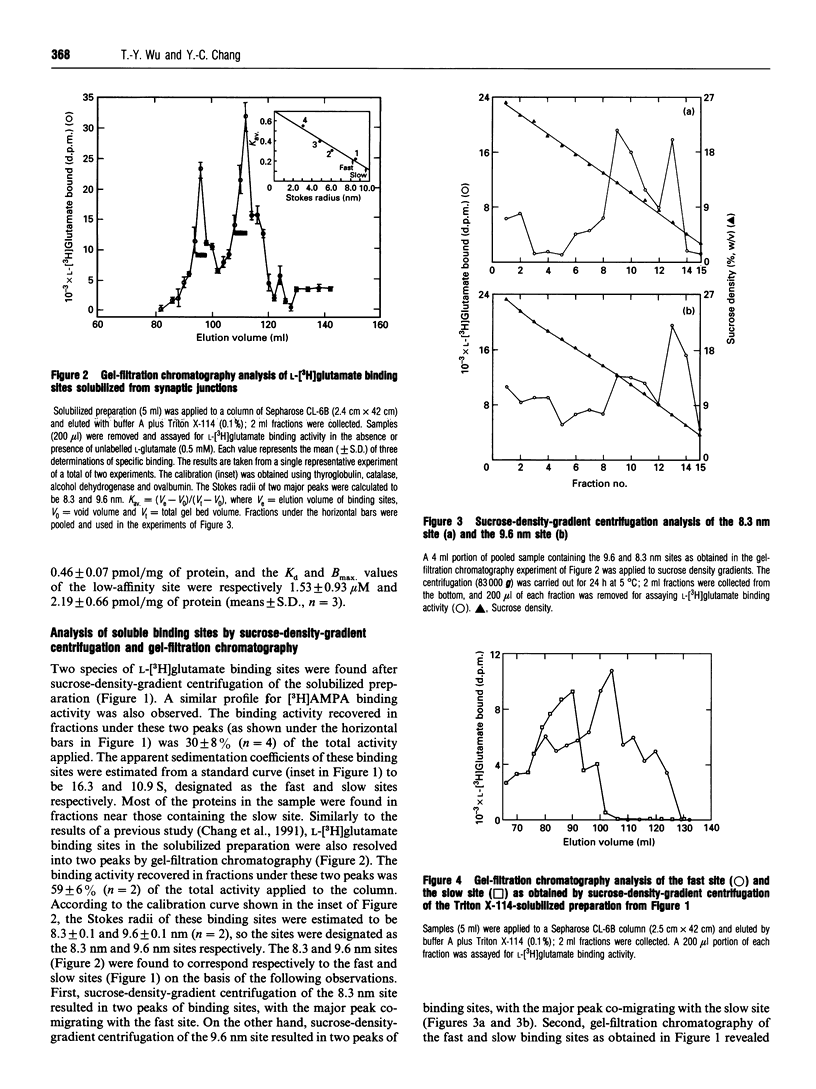
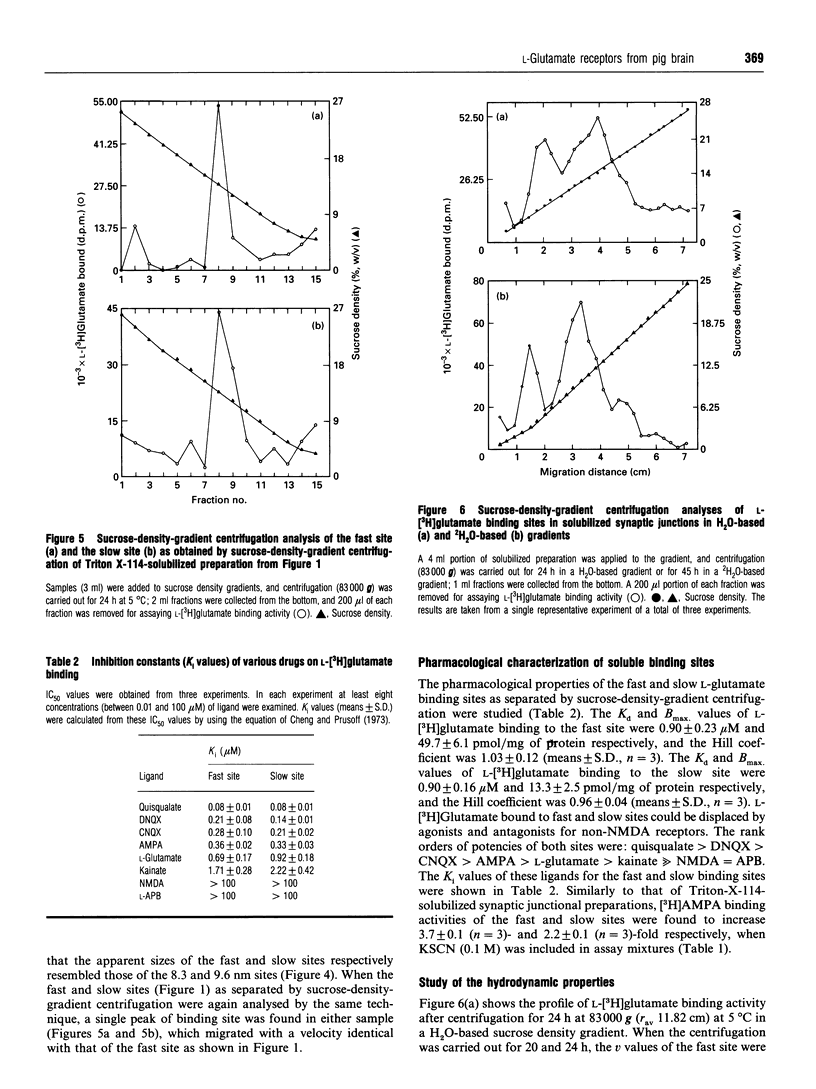
L-[3H]Glutamate binding sites with characteristics resembling that of membrane-bound alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate-subtype L-glutamate receptors have been solubilized from pig brain synaptic junctions by Triton X-114. Binding of [3H]AMPA to these soluble sites in the presence of KSCN results in a curvilinear Scatchard plot that can be resolved into a high-affinity component and a low-affinity component. These Triton-X-114-solubilized sites can be further separated into two species of binding sites by gel-filtration chromatography or sucrose-density-gradient centrifugation. The pharmacological profiles of these two species of binding site are almost identical, and the rank orders of potency for glutamatergic drugs in displacing L-[3H]glutamate binding to these sites are quisqualate > 6,7-dinitroquinoxaline-2,3-dione > 6-cyano-7-nitroquinoxaline-2,3-dione > AMPA > L-glutamate > kainate >> N-methyl-D-aspartate = L-2-amino-4-phosphonobutyrate. Both sites are found to bind [3H]AMPA, and in the presence of KSCN the binding activities are significantly enhanced. Analysis of the hydrodynamic behaviour of these binding sites by sucrose-density-gradient centrifugation in H2O- and 2H2O-based solvents and gel-filtration chromatography has revealed that one of these sites (Stokes radius 8.3 nm, sedimentation coefficient 18.5 S) consists of 562 kDa protein and 281 kDa detergent, and the other site (Stokes radius 9.6 nm, sedimentation coefficient 13.4 S) consists of 352 kDa protein and 569 kDa detergent. Frictional coefficients of these sites indicate that these receptor-detergent complexes are asymmetrical in structure, consistent with large transmembrane proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bettler B., Egebjerg J., Sharma G., Pecht G., Hermans-Borgmeyer I., Moll C., Stevens C. F., Heinemann S. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron. 1992 Feb;8(2):257–265. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- Blackstone C. D., Moss S. J., Martin L. J., Levey A. I., Price D. L., Huganir R. L. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992 Mar;58(3):1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Lawson-Wendling K., Pugsley T. A. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983 Jul 1;132(1):74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Lin Y. H., Lee Y. H., Leng C. H. Solubilization, characterization, and partial purification of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-, quisqualate-, kainate-sensitive L-glutamate binding sites from porcine brain synaptic junctions. J Neurochem. 1991 Dec;57(6):1921–1926. doi: 10.1111/j.1471-4159.1991.tb06404.x. [DOI] [PubMed] [Google Scholar]
- Chen J. W., Cunningham M. D., Galton N., Michaelis E. K. Immune labeling and purification of a 71-kDa glutamate-binding protein from brain synaptic membranes. Possible relationship of this protein to physiologic glutamate receptors. J Biol Chem. 1988 Jan 5;263(1):417–426. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clarke S., Smigel M. D. Size and shape of membrane protein-detergent complexes: hydrodynamic studies. Methods Enzymol. 1989;172:696–709. doi: 10.1016/s0076-6879(89)72039-5. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Mena E. E., Fagg G. E., Cotman C. W. Glutamate and aspartate binding sites are enriched in synaptic junctions isolated from rat brain. J Neurosci. 1981 Jun;1(6):620–625. doi: 10.1523/JNEUROSCI.01-06-00620.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. A., Kessler M., Lynch G. Evidence that high- and low-affinity DL-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) binding sites reflect membrane-dependent states of a single receptor. J Neurochem. 1992 Dec;59(6):1997–2004. doi: 10.1111/j.1471-4159.1992.tb10086.x. [DOI] [PubMed] [Google Scholar]
- Hampson D. R., Huie D., Wenthold R. J. Solubilization of kainic acid binding sites from rat brain. J Neurochem. 1987 Oct;49(4):1209–1215. doi: 10.1111/j.1471-4159.1987.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Hampson D. R., Wenthold R. J. A kainic acid receptor from frog brain purified using domoic acid affinity chromatography. J Biol Chem. 1988 Feb 15;263(5):2500–2505. [PubMed] [Google Scholar]
- Henley J. M., Barnard E. A. Solubilisation and characterisation of a putative quisqualate-type glutamate receptor from chick brain. J Neurochem. 1989 Jul;53(1):140–148. doi: 10.1111/j.1471-4159.1989.tb07305.x. [DOI] [PubMed] [Google Scholar]
- Henley J. M., Nielsen M., Barnard E. A. Characterisation of an allosteric modulatory protein associated with alpha-[3H]amino-3-hydroxy-5-methylisoxazolepropionate binding sites in chick telencephalon: effects of high-energy radiation and detergent solubilisation. J Neurochem. 1992 Jun;58(6):2030–2036. doi: 10.1111/j.1471-4159.1992.tb10943.x. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Drejer J. Chaotropic ions affect the conformation of quisqualate receptors in rat cortical membranes. J Neurochem. 1988 Aug;51(2):457–461. doi: 10.1111/j.1471-4159.1988.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Honoré T., Nielsen M. Complex structure of quisqualate-sensitive glutamate receptors in rat cortex. Neurosci Lett. 1985 Feb 28;54(1):27–32. doi: 10.1016/s0304-3940(85)80113-0. [DOI] [PubMed] [Google Scholar]
- Hunter C., Wheaton K. D., Wenthold R. J. Solubilization and partial purification of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid binding sites from rat brain. J Neurochem. 1990 Jan;54(1):118–125. doi: 10.1111/j.1471-4159.1990.tb13290.x. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Baughman R. W. Both NMDA and non-NMDA subtypes of glutamate receptors are concentrated at synapses on cerebral cortical neurons in culture. Neuron. 1991 Oct;7(4):593–603. doi: 10.1016/0896-6273(91)90372-7. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kuonen D. R., Roberts P. J. Solubilisation of a glutamate binding protein from rat brain. J Neurochem. 1987 Jul;49(1):272–281. doi: 10.1111/j.1471-4159.1987.tb03426.x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Michaelis E. K., Michaelis M. L., Stormann T. M., Chittenden W. L., Grubbs R. D. Purification and molecular characterization of the brain synaptic membrane glutamate-binding protein. J Neurochem. 1983 Jun;40(6):1742–1753. doi: 10.1111/j.1471-4159.1983.tb08150.x. [DOI] [PubMed] [Google Scholar]
- Michaelis E. K. Partial purification and characterization of a glutamate-binding membrane glycoprotein from rat brain. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1004–1012. doi: 10.1016/s0006-291x(75)80485-2. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Sakimura K., Bujo H., Kushiya E., Araki K., Yamazaki M., Yamazaki M., Meguro H., Warashina A., Numa S., Mishina M. Functional expression from cloned cDNAs of glutamate receptor species responsive to kainate and quisqualate. FEBS Lett. 1990 Oct 15;272(1-2):73–80. doi: 10.1016/0014-5793(90)80452-o. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Swope S. L., Moss S. J., Blackstone C. D., Huganir R. L. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 1992 May;6(8):2514–2523. [PubMed] [Google Scholar]
- Vyklicky L., Jr, Patneau D. K., Mayer M. L. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron. 1991 Dec;7(6):971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Yokotani N., Doi K., Wada K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J Biol Chem. 1992 Jan 5;267(1):501–507. [PubMed] [Google Scholar]
- Zorumski C. F., Thio L. L., Clark G. D., Clifford D. B. Blockade of desensitization augments quisqualate excitotoxicity in hippocampal neurons. Neuron. 1990 Jul;5(1):61–66. doi: 10.1016/0896-6273(90)90033-c. [DOI] [PubMed] [Google Scholar]


