Abstract
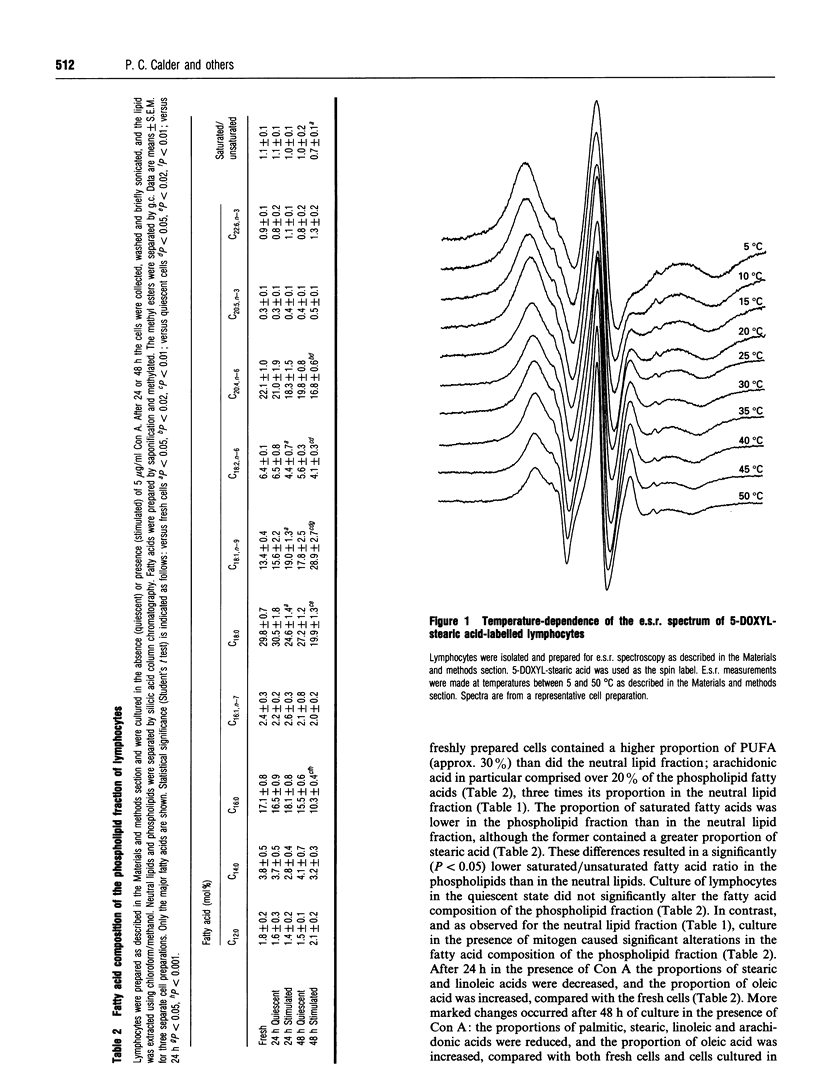
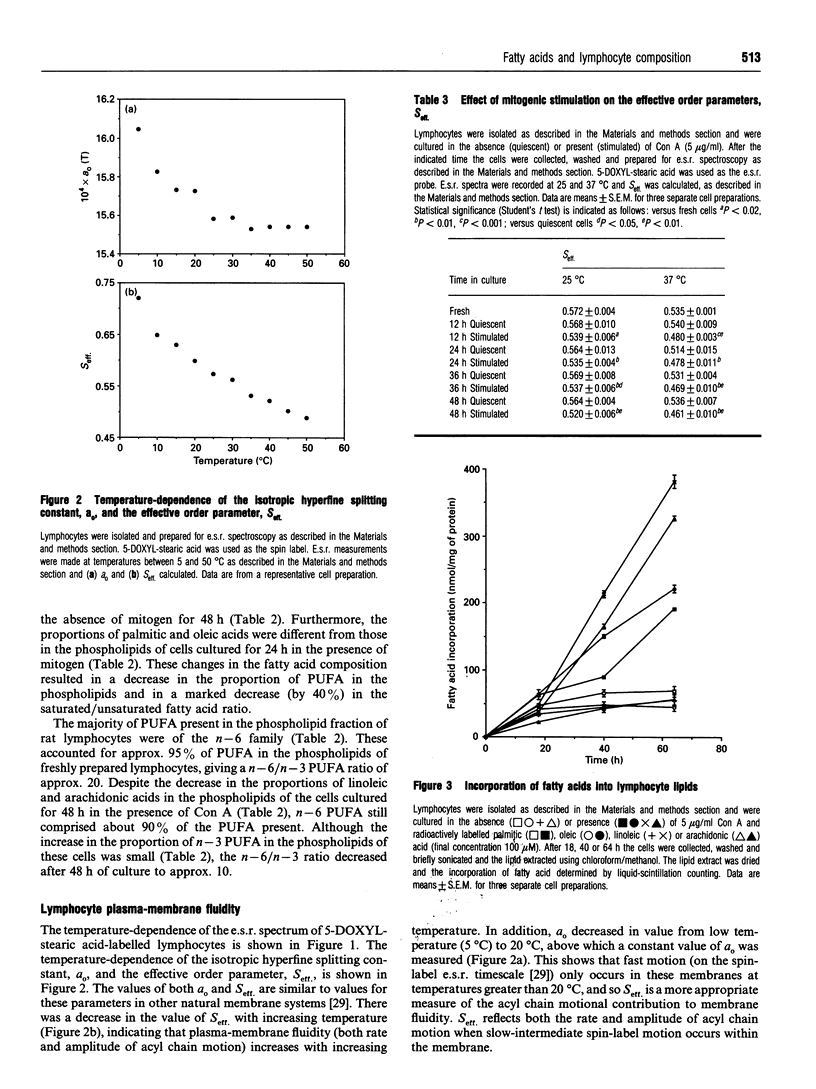
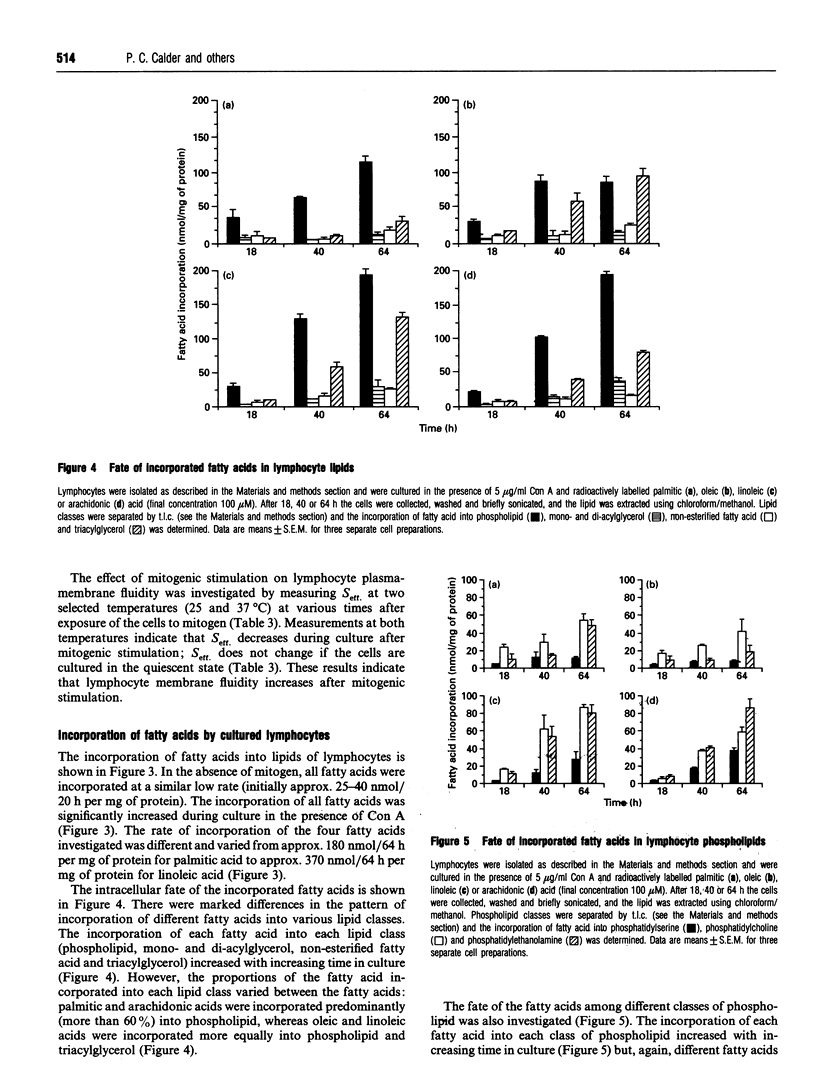
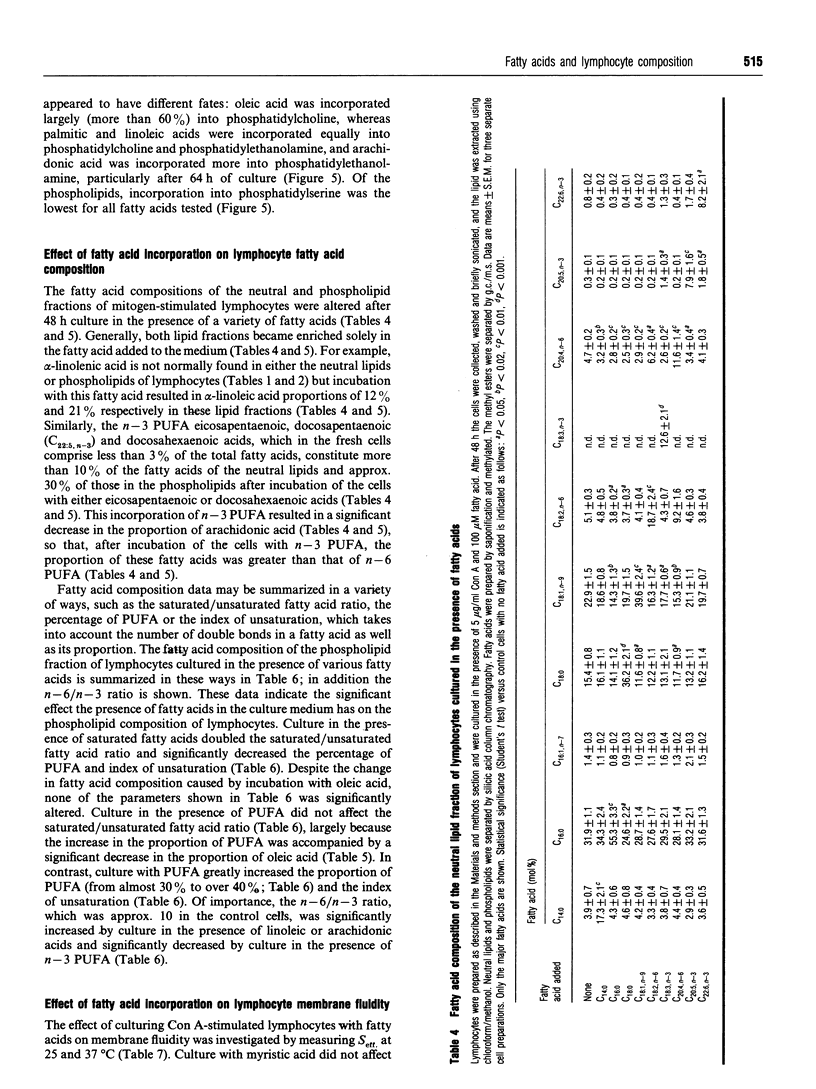
The fatty acid compositions of the neutral lipid and phospholipid fractions of rat lymph node lymphocytes were characterized. Stimulation of rat lymphocytes with the T-cell mitogen concanavalin A resulted in significant changes in the fatty acid composition of both neutral lipids and phospholipids (a decrease in the proportions of stearic, linoleic and arachidonic acids and an increase in the proportion of oleic acid). Membrane fluidity was measured using nitroxide spin-label e.s.r., and increased during culture with concanavalin A. Culturing the lymphocytes in the absence of mitogen did not affect fatty acid composition or membrane fluidity. The uptake and fate of palmitic, oleic, linoleic and arachidonic acids were studied in detail; there was a time-dependent incorporation of each fatty acid into all lipid classes but each fatty acid had a characteristic fate. Palmitic and arachidonic acids were incorporated principally into phospholipids whereas oleic and linoleic acids were incorporated in similar proportions into phospholipids and triacylglycerols. Oleic acid was incorporated mainly into phosphatidylcholine, palmitic and linoleic acids were incorporated equally into phosphatidylcholine and phosphatidylethanolamine, and arachidonic acid was incorporated mainly into phosphatidylethanolamine. Supplementation of the culture medium with particular fatty acids (myristic, palmitic, stearic, oleic, linoleic, alpha-linolenic, arachidonic, eicosapentaenoic or docosahexaenoic acid) led to enrichment of that fatty acid in both neutral lipids and phospholipids. This generated lymphocytes with phospholipids differing in saturated/unsaturated fatty acid ratio, degree of polyunsaturation, index of unsaturation and n - 6/n - 3 ratio. This method allowed the introduction into lymphocyte phospholipids of fatty acids not normally present (e.g. alpha-linolenic) or usually present in low proportions (eicosapentaenoic and docosahexaenoic). These three n - 3 polyunsaturated fatty acids replaced arachidonic acid in lymphocyte phospholipids. Fatty acid incorporation led to an alteration in lymphocyte membrane fluidity: palmitic and stearic acids decreased fluidity whereas the unsaturated fatty acids increased fluidity. It is proposed that the changes in lymphocyte phospholipid fatty acid composition and membrane fluidity brought about by culture in the presence of polyunsaturated fatty acids are responsible for the inhibition of lymphocyte functions caused by these fatty acids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anel A., Naval J., González B., Torres J. M., Mishal Z., Uriel J., Piñeiro A. Fatty acid metabolism in human lymphocytes. I. Time-course changes in fatty acid composition and membrane fluidity during blastic transformation of peripheral blood lymphocytes. Biochim Biophys Acta. 1990 Jun 14;1044(3):323–331. doi: 10.1016/0005-2760(90)90076-a. [DOI] [PubMed] [Google Scholar]
- Anel A., Naval J., González B., Uriel J., Piñeiro A. Fatty acid metabolism in human lymphocytes. II. Activation of fatty acid desaturase-elongase systems during blastic transformation. Biochim Biophys Acta. 1990 Jun 14;1044(3):332–339. doi: 10.1016/0005-2760(90)90077-b. [DOI] [PubMed] [Google Scholar]
- Barnett R. E., Scott R. E., Furcht L. T., Kersey J. H. Evidence that mitogenic lectins induce changes in lymphocyte membrane fluidity. Nature. 1974 May 31;249(456):465–466. doi: 10.1038/249465a0. [DOI] [PubMed] [Google Scholar]
- Bates D., Cartlidge N. E., French J. M., Jackson M. J., Nightingale S., Shaw D. A., Smith S., Woo E., Hawkins S. A., Millar J. H. A double-blind controlled trial of long chain n-3 polyunsaturated fatty acids in the treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):18–22. doi: 10.1136/jnnp.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittiner S. B., Tucker W. F., Cartwright I., Bleehen S. S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988 Feb 20;1(8582):378–380. doi: 10.1016/s0140-6736(88)91181-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner R. R. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog Lipid Res. 1984;23(2):69–96. doi: 10.1016/0163-7827(84)90008-0. [DOI] [PubMed] [Google Scholar]
- Calder P. C., Bevan S. J., Newsholme E. A. The inhibition of T-lymphocyte proliferation by fatty acids is via an eicosanoid-independent mechanism. Immunology. 1992 Jan;75(1):108–115. [PMC free article] [PubMed] [Google Scholar]
- Calder P. C., Bond J. A., Bevan S. J., Hunt S. V., Newsholme E. A. Effect of fatty acids on the proliferation of concanavalin A-stimulated rat lymph node lymphocytes. Int J Biochem. 1991;23(5-6):579–588. doi: 10.1016/0020-711x(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Calder P. C., Bond J. A., Harvey D. J., Gordon S., Newsholme E. A. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990 Aug 1;269(3):807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C., Newsholme E. A. Polyunsaturated fatty acids suppress human peripheral blood lymphocyte proliferation and interleukin-2 production. Clin Sci (Lond) 1992 Jun;82(6):695–700. doi: 10.1042/cs0820695. [DOI] [PubMed] [Google Scholar]
- Cherenkevich S. N., Vanderkooi J. M., Deutsch C. Changes in membrane fluidity associated with lymphocyte stimulation by succinyl-concanavalin A. Biochim Biophys Acta. 1982 Apr 7;686(2):170–176. doi: 10.1016/0005-2736(82)90109-2. [DOI] [PubMed] [Google Scholar]
- Collard J. G., De Wildt A., Oomen-Meulemans E. P., Smeekens J., Emmelot P. Increase in fluidity of membrane lipids in lymphocytes, fibroblasts and liver cells stimulated for growth. FEBS Lett. 1977 May 15;77(2):173–178. doi: 10.1016/0014-5793(77)80228-7. [DOI] [PubMed] [Google Scholar]
- Curtain C. C., Looney F. D., Marchalonis J. J., Raison J. K. Changes in lipid ordering and state of aggregation in lymphocyte plasma membranes after exposure to mitogens. J Membr Biol. 1978 Dec 29;44(3-4):211–232. doi: 10.1007/BF01944222. [DOI] [PubMed] [Google Scholar]
- Ferber E., De Pasquale G. G., Resch K. Phospholipid metabolism of stimulated lymphocytes. Composition of phospholipid fatty acids. Biochim Biophys Acta. 1975 Sep 19;398(3):364–376. doi: 10.1016/0005-2760(75)90187-3. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Khan N. R., Trew-Marshall B. J., Cupples C. G., Mély-Goubert B. Early biochemical events in lymphocyte activation. II. Selectivity of A23187 for T lymphocytes and the use of an apolar fluorescent probe (1,6-diphenyl-1,3,5-hexatriene) to monitor ionophore- and lectin-induced lymphocyte activation. Cell Immunol. 1981 Feb;58(1):134–146. doi: 10.1016/0008-8749(81)90155-6. [DOI] [PubMed] [Google Scholar]
- Goppelt-Strübe M., Resch K. Polyunsaturated fatty acids are enriched in the plasma membranes of mitogen-stimulated T-lymphocytes. Biochim Biophys Acta. 1987 Nov 2;904(1):22–28. doi: 10.1016/0005-2736(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Hadden J. W. Transmembrane signals in the activation of T lymphocytes by mitogenic antigens. Immunol Today. 1988 Jul-Aug;9(7-8):235–239. doi: 10.1016/0167-5699(88)91222-4. [DOI] [PubMed] [Google Scholar]
- Harris J., Power T. J., Bieber A. L., Watts A. An electron-spin-resonance spin-label study of the interaction of purified Mojave toxin with synaptosomal membranes from rat brain. Eur J Biochem. 1983 Apr 5;131(3):559–565. doi: 10.1111/j.1432-1033.1983.tb07299.x. [DOI] [PubMed] [Google Scholar]
- Hwang D. Essential fatty acids and immune response. FASEB J. 1989 Jul;3(9):2052–2061. doi: 10.1096/fasebj.3.9.2501132. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M. Decrease in microviscosity of lymphocyte surface membrane associated with stimulation induced by concanavalin A. Eur J Immunol. 1975 Mar;5(3):166–170. doi: 10.1002/eji.1830050303. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Jubiz W., Michalek A., Rynes R. I., Bartholomew L. E., Bigaouette J., Timchalk M., Beeler D., Lininger L. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987 Apr;106(4):497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- Murphy M. G. Dietary fatty acids and membrane protein function. J Nutr Biochem. 1990 Feb;1(2):68–79. doi: 10.1016/0955-2863(90)90052-m. [DOI] [PubMed] [Google Scholar]
- Parola A. H., Kaplan J. H., Lockwood S. H., Uzgiris E. E. Activation of human lymphocytes by concanavalin A or purified protein derivative results in no alteration of fluorescence polarization of lipid probes although the electrophoretic mobility of the cells is changed. Biochim Biophys Acta. 1981 Dec 21;649(3):616–624. doi: 10.1016/0005-2736(81)90166-8. [DOI] [PubMed] [Google Scholar]
- Resch K., Ferber E., Odenthal J., Fischer H. Early changes in the phospholipid metabolism of lymphocytes following stimulation with phytohemagglutinin and with lysolecithin. Eur J Immunol. 1971 Jun;1(3):162–165. doi: 10.1002/eji.1830010304. [DOI] [PubMed] [Google Scholar]
- Resch K., Ferber E. Phospholipid metabolism of stimulated lymphocytes. Effects of phytohemagglutinin, concanavalin A and anti-immunoglobulin serum. Eur J Biochem. 1972 May;27(1):153–161. doi: 10.1111/j.1432-1033.1972.tb01821.x. [DOI] [PubMed] [Google Scholar]
- Resch K., Gelfand E. W., Hansen K., Ferber E. Lymphocyte activation: rapid changes in the phospholipid metabolism of plasma membranes during stimulation. Eur J Immunol. 1972 Dec;2(6):598–601. doi: 10.1002/eji.1830020623. [DOI] [PubMed] [Google Scholar]
- Santoli D., Phillips P. D., Colt T. L., Zurier R. B. Suppression of interleukin 2-dependent human T cell growth in vitro by prostaglandin E (PGE) and their precursor fatty acids. Evidence for a PGE-independent mechanism of inhibition by the fatty acids. J Clin Invest. 1990 Feb;85(2):424–432. doi: 10.1172/JCI114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speizer L. A., Watson M. J., Brunton L. L. Differential effects of omega-3 fish oils on protein kinase activities in vitro. Am J Physiol. 1991 Jul;261(1 Pt 1):E109–E114. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Tsang W. M., Belin J., Smith A. D., Johnson S. M. Incubation of exogenous fatty acids with lymphocytes. Changes in fatty acid composition and effects on the rotational relaxation time of 1,6-diphenyl-1,3,5-hexatriene. Biochemistry. 1980 Jun 10;19(12):2756–2762. doi: 10.1021/bi00553a034. [DOI] [PubMed] [Google Scholar]
- Søyland E., Nenseter M. S., Braathen L., Drevon C. A. Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Invest. 1993 Feb;23(2):112–121. doi: 10.1111/j.1365-2362.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Toyoshima S., Osawa T. Lectins from Wistaria floribunda seeds and their effect on membrane fluidity of human peripheral lymphocytes. J Biol Chem. 1975 Mar 10;250(5):1655–1660. [PubMed] [Google Scholar]
- Weyman C., Morgan S. J., Belin J., Smith A. D. Phytohaemagglutinin stimulation of human lymphocytes: effect of fatty acids on uridine uptake and phosphoglyceride fatty acid profile. Biochim Biophys Acta. 1977 Jan 24;496(1):155–166. doi: 10.1016/0304-4165(77)90123-4. [DOI] [PubMed] [Google Scholar]


