Abstract
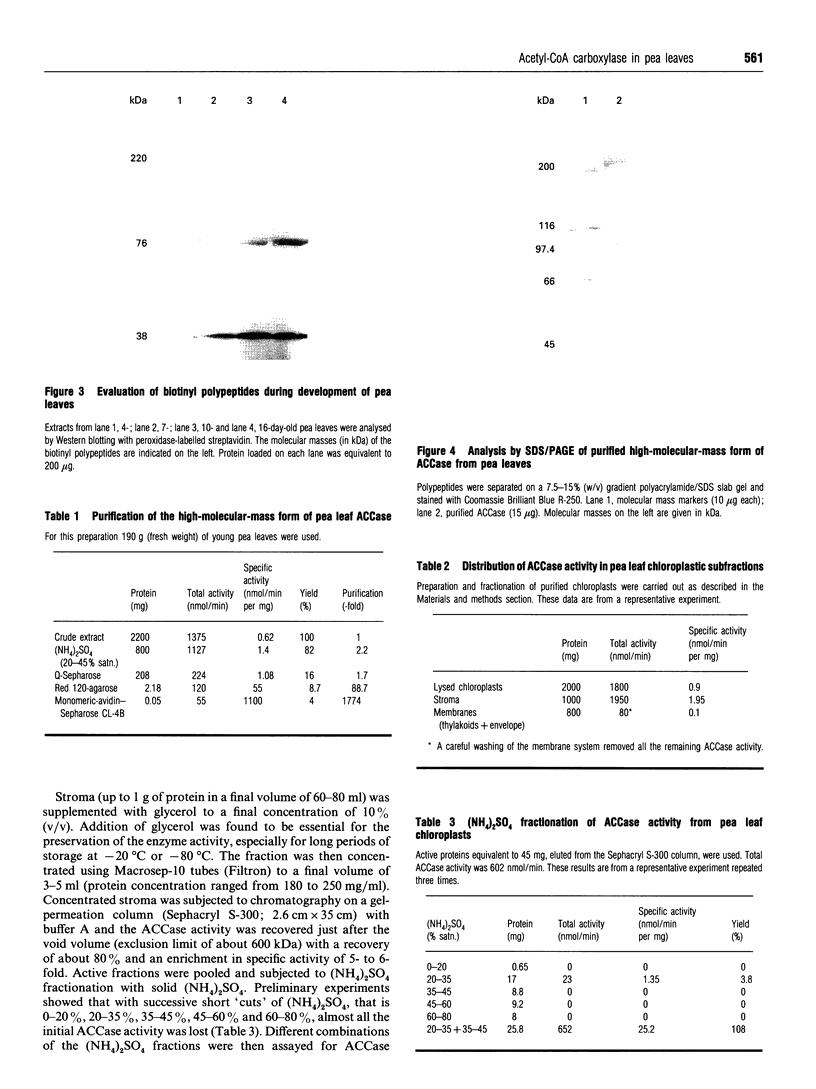
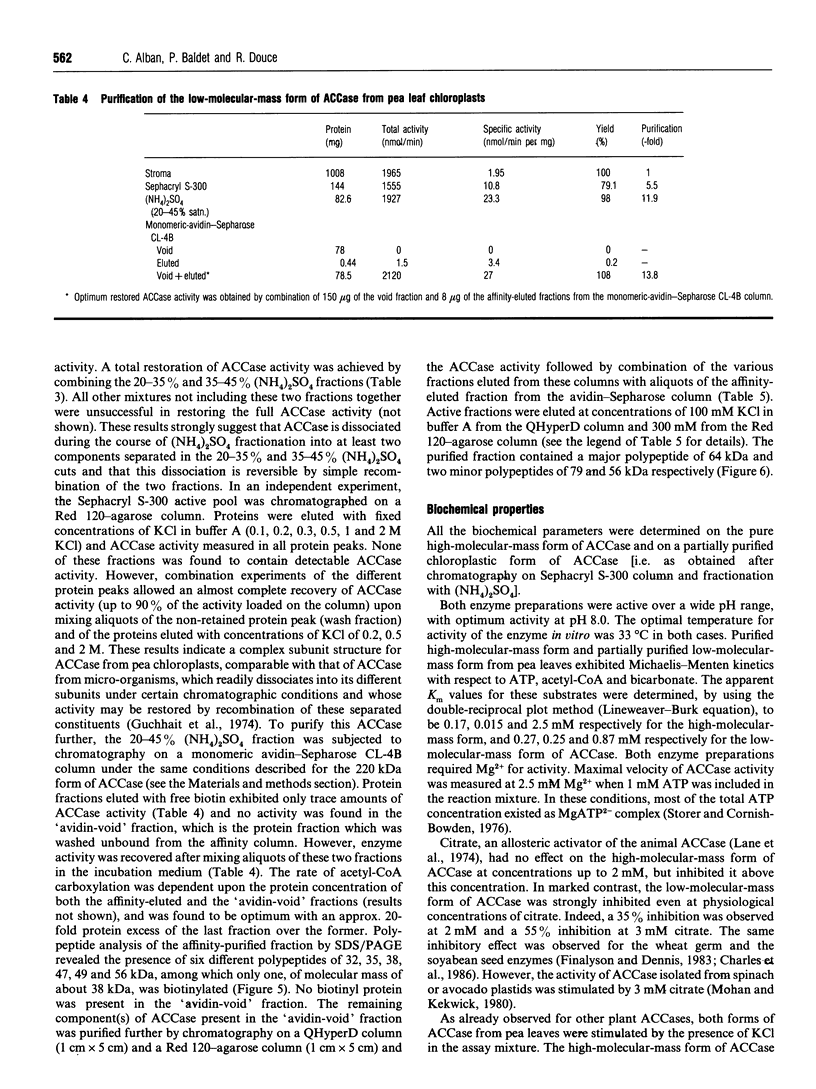
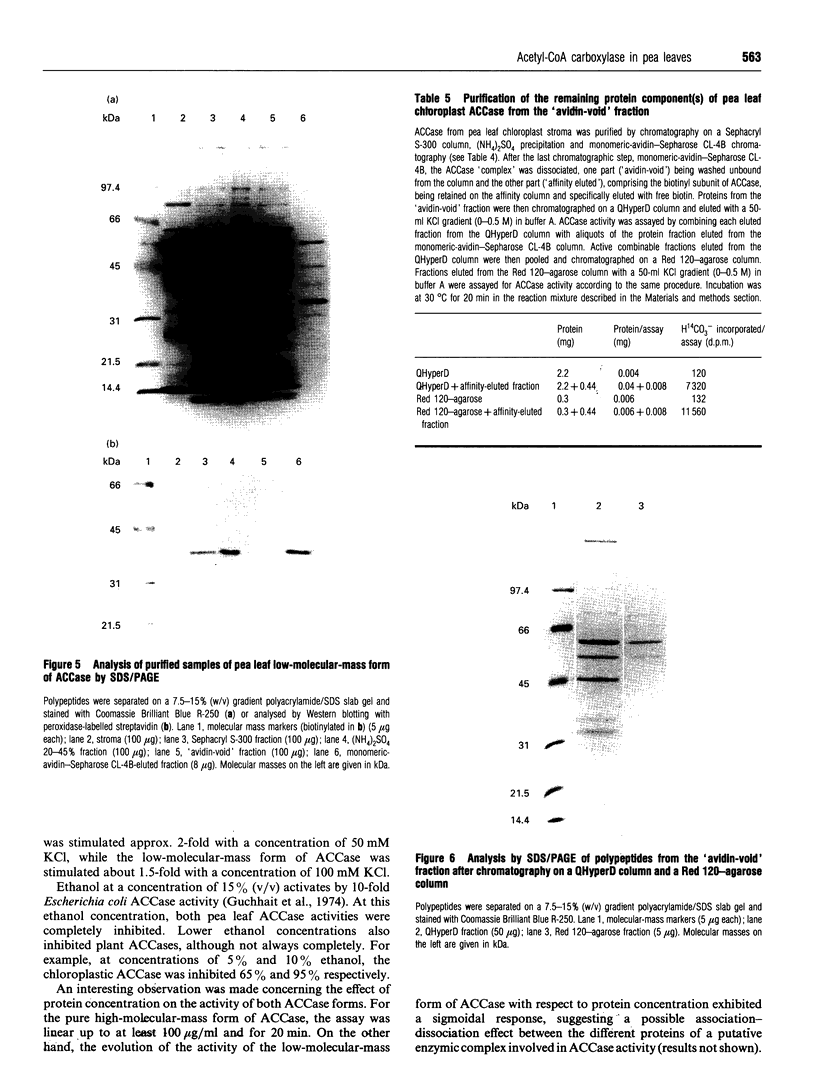
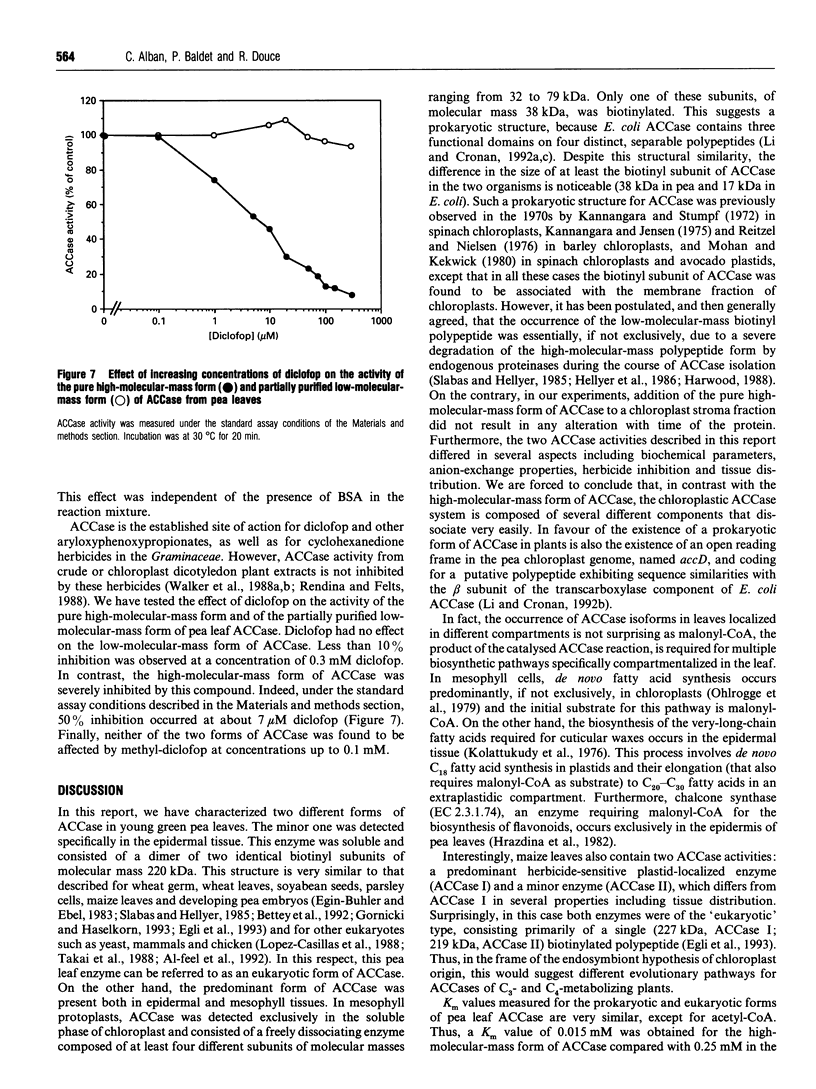
Young pea leaves contain two structurally different forms of acetyl-CoA carboxylase (EC 6.4.1.2; ACCase). A minor form, which accounted for about 20% of the total ACCase activity in the whole leaf, was detected in the epidermal tissue. This enzyme was soluble and was purified to homogeneity from young pea leaf extracts. It consisted of a dimer of two identical biotinyl subunits of molecular mass 220 kDa. In this respect, this multifunctional enzyme was comparable with that described in other plants and in other eukaryotes. A predominant form was present in both the epidermal and mesophyll tissues. In mesophyll protoplasts, ACCase was detected exclusively in the soluble phase of chloroplasts. This enzyme was partially purified from pea chloroplasts and consisted of a freely dissociating complex, the activity of which may be restored by combination of its separated constituents. The partially purified enzyme was composed of several subunits of molecular masses ranging from 32 to 79 kDa, for a native molecular mass > 600 kDa. One of these subunits, of molecular mass 38 kDa, was biotinylated. This complex subunit structure was comparable with that of microorganisms and was referred to as a 'prokaryotic' form of ACCase. Biochemical parameters were determined for both ACCase forms. Finally, both pea leaf ACCases exhibited different sensitivities towards the grass ACCase herbicide, diclofop. This compound had no effect on the 'prokaryotic' form of ACCase, while the 'eukaryotic' form was strongly inhibited.
Full text
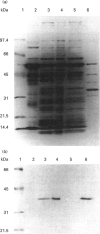
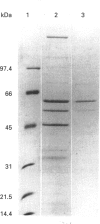
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Feel W., Chirala S. S., Wakil S. J. Cloning of the yeast FAS3 gene and primary structure of yeast acetyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4534–4538. doi: 10.1073/pnas.89.10.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban C., Baldet P., Axiotis S., Douce R. Purification and Characterization of 3-Methylcrotonyl-Coenzyme A Carboxylase from Higher Plant Mitochondria. Plant Physiol. 1993 Jul;102(3):957–965. doi: 10.1104/pp.102.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Baldet P., Alban C., Axiotis S., Douce R. Characterization of biotin and 3-methylcrotonyl-coenzyme a carboxylase in higher plant mitochondria. Plant Physiol. 1992 Jun;99(2):450–455. doi: 10.1104/pp.99.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
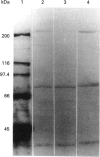
- Baldet P., Alban C., Axiotis S., Douce R. Localization of free and bound biotin in cells from green pea leaves. Arch Biochem Biophys. 1993 May 15;303(1):67–73. doi: 10.1006/abbi.1993.1256. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wurtele E. S., Wang X., Nikolau B. J. Purification and characterization of 3-methylcrotonyl-CoA carboxylase from somatic embryos of Daucus carota. Arch Biochem Biophys. 1993 Aug 15;305(1):103–109. doi: 10.1006/abbi.1993.1398. [DOI] [PubMed] [Google Scholar]
- Egin-Bühler B., Ebel J. Improved purification and further characterization of acetyl-CoA carboxylase from cultured cells of parsley (Petroselinum hortense). Eur J Biochem. 1983 Jun 15;133(2):335–339. doi: 10.1111/j.1432-1033.1983.tb07467.x. [DOI] [PubMed] [Google Scholar]
- Egli M. A., Gengenbach B. G., Gronwald J. W., Somers D. A., Wyse D. L. Characterization of Maize Acetyl-Coenzyme A Carboxylase. Plant Physiol. 1993 Feb;101(2):499–506. doi: 10.1104/pp.101.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson S. A., Dennis D. T. Acetyl-coenzyme A carboxylase from the developing endosperm of Ricinus communis. I. Isolation and characterization. Arch Biochem Biophys. 1983 Sep;225(2):576–585. doi: 10.1016/0003-9861(83)90069-3. [DOI] [PubMed] [Google Scholar]
- Gornicki P., Haselkorn R. Wheat acetyl-CoA carboxylase. Plant Mol Biol. 1993 Jun;22(3):547–552. doi: 10.1007/BF00015984. [DOI] [PubMed] [Google Scholar]
- Guchhait R. B., Polakis S. E., Dimroth P., Stoll E., Moss J., Lane M. D. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem. 1974 Oct 25;249(20):6633–6645. [PubMed] [Google Scholar]
- Hellyer A., Bambridge H. E., Slabas A. R. Plant acetyl-CoA carboxylase. Biochem Soc Trans. 1986 Jun;14(3):565–568. doi: 10.1042/bst0140565. [DOI] [PubMed] [Google Scholar]
- Henrikson K. P., Allen S. H., Maloy W. L. An avidin monomer affinity column for the purification of biotin-containing enzymes. Anal Biochem. 1979 Apr 15;94(2):366–370. doi: 10.1016/0003-2697(79)90374-9. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Marx G. A., Hoch H. C. Distribution of Secondary Plant Metabolites and Their Biosynthetic Enzymes in Pea (Pisum sativum L.) Leaves : Anthocyanins and Flavonol Glycosides. Plant Physiol. 1982 Sep;70(3):745–748. doi: 10.1104/pp.70.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Jensen C. J. Biotin carboxyl carrier protein in barley chloroplast membranes. Eur J Biochem. 1975 May;54(1):25–30. doi: 10.1111/j.1432-1033.1975.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LIV. A procaryotic type acetyl CoA carboxylase in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):83–91. doi: 10.1016/0003-9861(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. The mechanism of biotin-dependent enzymes. Annu Rev Biochem. 1989;58:195–221. doi: 10.1146/annurev.bi.58.070189.001211. [DOI] [PubMed] [Google Scholar]
- Kohanski R. A., Lane M. D. Monovalent avidin affinity columns. Methods Enzymol. 1990;184:194–200. doi: 10.1016/0076-6879(90)84274-k. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Moss J., Polakis S. E. Acetyl coenzyme A carboxylase. Curr Top Cell Regul. 1974;8(0):139–195. [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr Putative zinc finger protein encoded by a conserved chloroplast gene is very likely a subunit of a biotin-dependent carboxylase. Plant Mol Biol. 1992 Dec;20(5):759–761. doi: 10.1007/BF00027147. [DOI] [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Jan 15;267(2):855–863. [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Aug 25;267(24):16841–16847. [PubMed] [Google Scholar]
- López-Casillas F., Bai D. H., Luo X. C., Kong I. S., Hermodson M. A., Kim K. H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S. B., Kekwick R. G. Acetyl-coenzyme A carboxylase from avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem J. 1980 Jun 1;187(3):667–676. doi: 10.1042/bj1870667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau B. J., Hawke J. C. Purification and characterization of maize leaf acetyl-coenzyme A carboxylase. Arch Biochem Biophys. 1984 Jan;228(1):86–96. doi: 10.1016/0003-9861(84)90049-3. [DOI] [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Tissue distribution of acetyl-coenzyme a carboxylase in leaves. Plant Physiol. 1984 Aug;75(4):895–901. doi: 10.1104/pp.75.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller D., Roughan G., Ohlrogge J. B. Regulation of plant Fatty Acid biosynthesis : analysis of acyl-coenzyme a and acyl-acyl carrier protein substrate pools in spinach and pea chloroplasts. Plant Physiol. 1992 Oct;100(2):923–930. doi: 10.1104/pp.100.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel L., Nielsen N. C. Acetyl-CoA carboxylase during development of plastids in wild-type and mutant barley seedlings. Eur J Biochem. 1976 May 17;65(1):131–138. doi: 10.1111/j.1432-1033.1976.tb10397.x. [DOI] [PubMed] [Google Scholar]
- Rendina A. R., Felts J. M., Beaudoin J. D., Craig-Kennard A. C., Look L. L., Paraskos S. L., Hagenah J. A. Kinetic characterization, stereoselectivity, and species selectivity of the inhibition of plant acetyl-CoA carboxylase by the aryloxyphenoxypropionic acid grass herbicides. Arch Biochem Biophys. 1988 Aug 15;265(1):219–225. doi: 10.1016/0003-9861(88)90387-6. [DOI] [PubMed] [Google Scholar]
- Rendina A. R., Felts J. M. Cyclohexanedione Herbicides Are Selective and Potent Inhibitors of Acetyl-CoA Carboxylase from Grasses. Plant Physiol. 1988 Apr;86(4):983–986. doi: 10.1104/pp.86.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Yokoyama C., Wada K., Tanabe T. Primary structure of chicken liver acetyl-CoA carboxylase deduced from cDNA sequence. J Biol Chem. 1988 Feb 25;263(6):2651–2657. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K. A., Ridley S. M., Harwood J. L. Effects of the selective herbicide fluazifop on fatty acid synthesis in pea (Pisum sativum) and barley (Hordeum vulgare). Biochem J. 1988 Sep 15;254(3):811–817. doi: 10.1042/bj2540811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K. A., Ridley S. M., Lewis T., Harwood J. L. Fluazifop, a grass-selective herbicide which inhibits acetyl-CoA carboxylase in sensitive plant species. Biochem J. 1988 Aug 15;254(1):307–310. doi: 10.1042/bj2540307. [DOI] [PMC free article] [PubMed] [Google Scholar]