Abstract
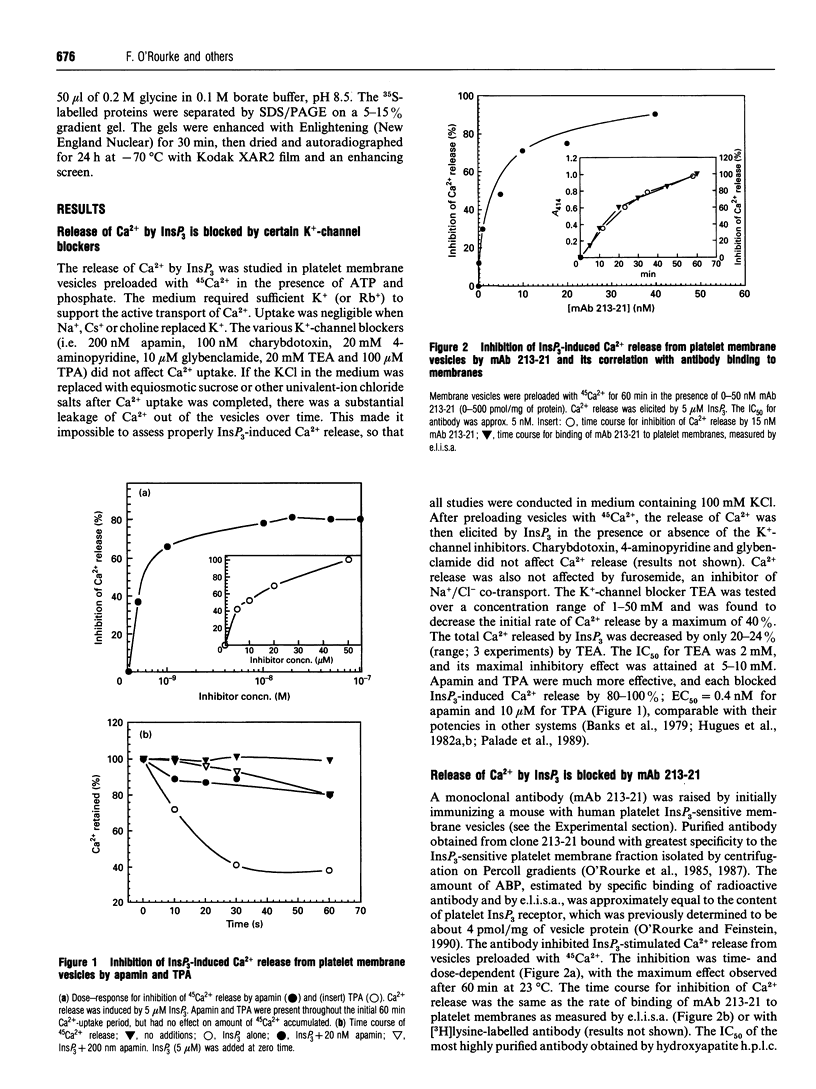
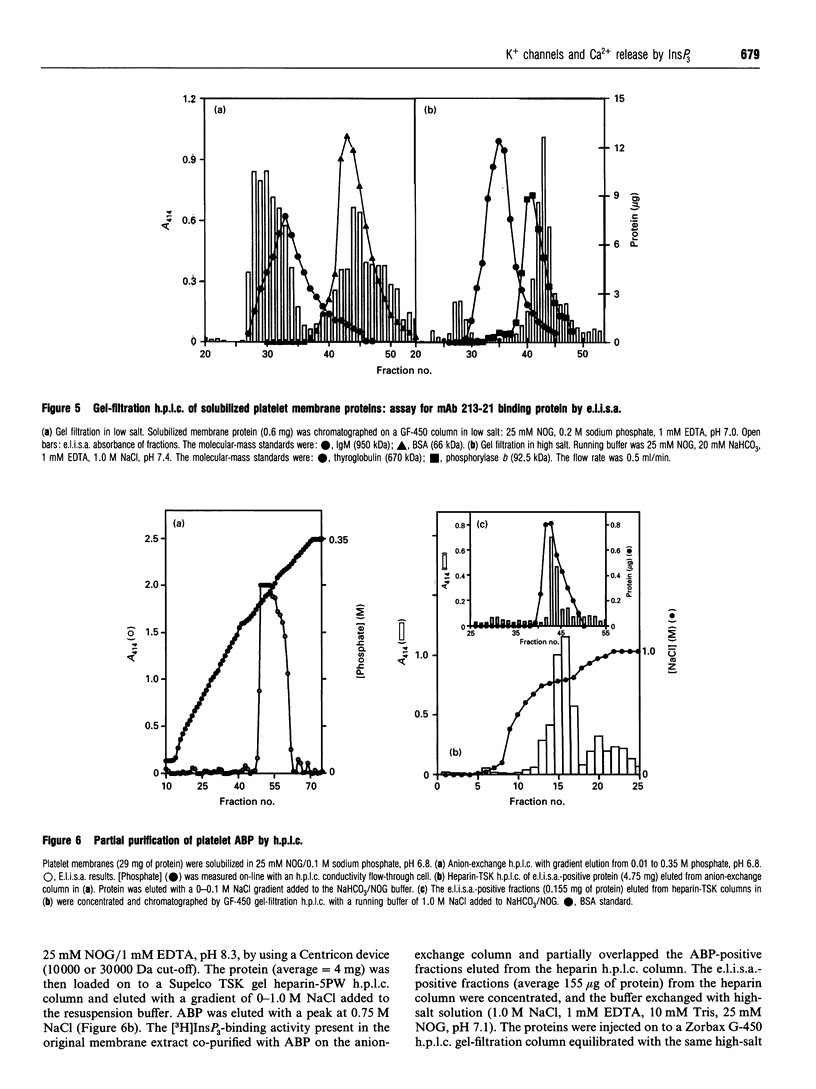
Ins(1,4,5)P3-induced Ca2+ release from platelet membrane vesicles was blocked by apamin, a selective inhibitor of low-conductance Ca(2+)-activated K+ channels, and by tetrapentylammonium ion, and was weakly inhibited by tetraethylammonium ion. Other K(+)-channel blockers, i.e. charybdotoxin, 4-aminopyridine and glybenclamide were ineffective. A monoclonal antibody (mAb 213-21) obtained by immunizing mice with the InsP3-sensitive membrane fraction from platelets also blocked Ca2+ release by InsP3 from membrane vesicles obtained from platelets, cerebellum, aortic smooth muscle, HEL cells and sea-urchin eggs. ATP-dependent Ca2+ uptake and binding of [3H]InsP3 to platelet membranes was unaffected by either K(+)-channel blockers or mAb 213-21. Blockade of Ca2+ release by apamin, tetrapentylammonium and mAb 213-21 was not affected by the Na+/H+ carrier monensin or the protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), but could be completely reversed by the K+/H+ ionophore nigericin and partially reversed by the K+ carrier valinomycin. The antibody-binding protein (ABP) solubilized from platelets, cerebellum, and smooth muscle chromatographed identically on gel filtration, anion-exchange and heparin-TSK h.p.l.c. ABP was purified to apparent homogeneity from platelets and aortic smooth muscle as a 63 kDa protein by immunoaffinity chromatography on mAb 213-21-agarose. These results suggest that optimal Ca2+ release by InsP3 from platelet membrane vesicles may require the tandem function of a K+ channel. A counterflow of K+ ions could prevent the build-up of a membrane potential (inside negative) that would tend to oppose Ca2+ release. The 63 kDa protein may function to regulate K+ permeability that is coupled to the Ca2+ efflux via the InsP3 receptor.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramcheck C. W., Best P. M. Physiological role and selectivity of the in situ potassium channel of the sarcoplasmic reticulum in skinned frog skeletal muscle fibers. J Gen Physiol. 1989 Jan;93(1):1–21. doi: 10.1085/jgp.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Baró I., Escande D. A long lasting Ca2+-activated outward current in guinea-pig atrial myocytes. Pflugers Arch. 1989 Oct;415(1):63–71. doi: 10.1007/BF00373142. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. B., Benevolensky D. S., Naumov A. P. Potassium channels in aortic microsomes: conductance, selectivity, barium-induced blockage and subconductance states. Biochim Biophys Acta. 1991 Apr 26;1064(1):75–80. doi: 10.1016/0005-2736(91)90413-3. [DOI] [PubMed] [Google Scholar]
- Bkaily G., Sperelakis N., Renaud J. F., Payet M. D. Apamin, a highly specific Ca2+ blocking agent in heart muscle. Am J Physiol. 1985 Jun;248(6 Pt 2):H961–H965. doi: 10.1152/ajpheart.1985.248.6.H961. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Saito A., Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Fine B. P., Hansen K. A., Salcedo J. R., Aviv A. Calcium-activated potassium channels in human platelets. Proc Soc Exp Biol Med. 1989 Nov;192(2):109–113. doi: 10.3181/00379727-192-42963. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G. Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987 Aug;409(4-5):374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Lewis R. S., Cahalan M. D. Ca(2+)-activated K+ channels in human leukemic T cells. J Gen Physiol. 1992 Jan;99(1):63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues M., Schmid H., Romey G., Duval D., Frelin C., Lazdunski M. The Ca2+-dependent slow K+ conductance in cultured rat muscle cells: characterization with apamin. EMBO J. 1982;1(9):1039–1042. doi: 10.1002/j.1460-2075.1982.tb01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Morita T., Kawasaki T., Taguchi T., Kasai M. Purification of a K(+)-channel protein of sarcoplasmic reticulum by assaying the channel activity in the planar lipid bilayer system. Biochim Biophys Acta. 1991 Aug 26;1067(2):213–220. doi: 10.1016/0005-2736(91)90046-b. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Kim D. H., Ohnishi S. T., Ikemoto N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J Biol Chem. 1983 Aug 25;258(16):9662–9668. [PubMed] [Google Scholar]
- Labbé-Jullié C., Granier C., Albericio F., Defendini M. L., Ceard B., Rochat H., Van Rietschoten J. Binding and toxicity of apamin. Characterization of the active site. Eur J Biochem. 1991 Mar 28;196(3):639–645. doi: 10.1111/j.1432-1033.1991.tb15860.x. [DOI] [PubMed] [Google Scholar]
- Leveque C., Marqueze B., Couraud F., Seagar M. Polypeptide components of the apamin receptor associated with a calcium activated potassium channel. FEBS Lett. 1990 Nov 26;275(1-2):185–189. doi: 10.1016/0014-5793(90)81468-4. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Lai F. A., Shen W. K., Meissner G., Strauss H. C. Reconstitution of the solubilized cardiac sarcoplasmic reticulum potassium channel. Identification of a putative Mr approximately 80 kDa polypeptide constituent. FEBS Lett. 1991 Oct 7;291(1):13–16. doi: 10.1016/0014-5793(91)81092-m. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Strauss H. C. Blockade of cardiac sarcoplasmic reticulum K+ channel by Ca2+: two-binding-site model of blockade. Biophys J. 1991 Jul;60(1):198–203. doi: 10.1016/S0006-3495(91)82043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrleitner M., Chadwick C. C., Timerman A. P., Fleischer S., Schindler H. Purified IP3 receptor from smooth muscle forms an IP3 gated and heparin sensitive Ca2+ channel in planar bilayers. Cell Calcium. 1991 Jul;12(7):505–514. doi: 10.1016/0143-4160(91)90032-a. [DOI] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium action potential and a prolonged calcium dependent after-hyperpolarization in mouse neuroblastoma cells. J Physiol. 1979 Jul;292:297–306. doi: 10.1113/jphysiol.1979.sp012851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium current and the activation of a slow potassium conductance in voltage-clamped mouse neuroblastoma cells. J Physiol. 1979 Jul;292:307–323. doi: 10.1113/jphysiol.1979.sp012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama A., Shimada K., Yamamoto T. Modification of immunoglobulin G using specific reactivity of sugar moiety. Immunochemistry. 1978 Aug;15(8):523–528. doi: 10.1016/0161-5890(78)90003-2. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- O'Rourke F., Feinstein M. B. The inositol 1,4,5-trisphosphate receptor binding sites of platelet membranes. pH-dependency, inhibition by polymeric sulphates, and the possible presence of arginine at the binding site. Biochem J. 1990 Apr 15;267(2):297–302. doi: 10.1042/bj2670297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke F., Zavoico G. B., Feinstein M. B. Release of Ca2+ by inositol 1,4,5-trisphosphate in platelet membrane vesicles is not dependent on cyclic AMP-dependent protein kinase. Biochem J. 1989 Feb 1;257(3):715–721. doi: 10.1042/bj2570715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke F., Zavoico G. B., Smith L. H., Jr, Feinstein M. B. Stimulus-response coupling in a cell-free platelet membrane system. GTP-dependent release of Ca2+ by thrombin, and inhibition by pertussis toxin and a monoclonal antibody that blocks calcium release by IP3. FEBS Lett. 1987 Apr 6;214(1):176–180. doi: 10.1016/0014-5793(87)80037-6. [DOI] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Volpe P., Alderson B., Otero A. S. Direct inhibition of inositol-1,4,5-trisphosphate-induced Ca2+ release from brain microsomes by K+ channel blockers. Mol Pharmacol. 1989 Oct;36(4):664–672. [PubMed] [Google Scholar]
- Rane S. G. A Ca2(+)-activated K+ current in ras-transformed fibroblasts is absent from nontransformed cells. Am J Physiol. 1991 Jan;260(1 Pt 1):C104–C112. doi: 10.1152/ajpcell.1991.260.1.C104. [DOI] [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Hugues M., Norman R., Ellory C., Borsotto M., Lazdunski M. Molecular properties of the apamin-binding component of the Ca2+-dependent K+ channel. Radiation-inactivation, affinity labelling and solubilization. Eur J Biochem. 1984 Jul 2;142(1):1–6. doi: 10.1111/j.1432-1033.1984.tb08242.x. [DOI] [PubMed] [Google Scholar]
- Seagar M. J., Marqueze B., Couraud F. Solubilization of the apamin receptor associated with a calcium-activated potassium channel from rat brain. J Neurosci. 1987 Feb;7(2):565–570. doi: 10.1523/JNEUROSCI.07-02-00565.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J., Cohen R. S., Pant H. C. Inositol trisphosphate-induced calcium release in brain microsomes. Brain Res. 1987 Sep 1;419(1-2):1–6. doi: 10.1016/0006-8993(87)90562-2. [DOI] [PubMed] [Google Scholar]
- Shah J., Pant H. C. Potassium-channel blockers inhibit inositol trisphosphate-induced calcium release in the microsomal fractions isolated from the rat brain. Biochem J. 1988 Mar 1;250(2):617–620. doi: 10.1042/bj2500617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. K., Hill J. A., Jr, Rasmusson R., Strauss H. C. Reconstitution of the K channel of cardiac sarcoplasmic reticulum. Prog Clin Biol Res. 1990;334:205–230. [PubMed] [Google Scholar]
- Sokabe M., Kasai M., Nomura K., Naruse K. Electrophysiological analysis of structural aspects of voltage-dependent SR K+ channel. Comp Biochem Physiol C. 1991;98(1):23–30. [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Uehara A., Yasukohchi M., Ogata S., Imanaga I. Activation by intracellular calcium of a potassium channel in cardiac sarcoplasmic reticulum. Pflugers Arch. 1991 Feb;417(6):651–653. doi: 10.1007/BF00372965. [DOI] [PubMed] [Google Scholar]
- Watras J., Benevolensky D. Inositol 1,4,5-trisphosphate-induced calcium release from canine aortic sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1987 Dec 10;931(3):354–363. doi: 10.1016/0167-4889(87)90227-8. [DOI] [PubMed] [Google Scholar]
- Wong B. S., Binstock L. Inhibition of potassium conductance with external tetraethylammonium ion in Myxicola giant axons. Biophys J. 1980 Dec;32(3):1037–1042. doi: 10.1016/S0006-3495(80)85034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



