Abstract
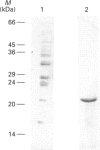
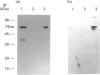
A luminally located peptidyl prolyl cis-trans-isomerase (PPI) has been purified from bovine liver microsomes. It has a molecular mass of 20.6 kDa, and N-terminal sequencing demonstrates strong sequence similarity to the sequences of the cyclophilin B family. The enzyme catalyses the isomerization of the standard proline-containing peptide N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide, as well as the refolding of RNAase T1. Kinetic properties, substrate-specificity data and inhibition by cyclosporin A indicate that it is a cyclophilin-type PPI, consistent with the amino-acid-sequence results.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber S., Krause K. H., Caroni P. s-cyclophilin is retained intracellularly via a unique COOH-terminal sequence and colocalizes with the calcium storage protein calreticulin. J Cell Biol. 1992 Jan;116(1):113–125. doi: 10.1083/jcb.116.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Freedman R. B. Peptidyl prolyl cis-trans-isomerase activity associated with the lumen of the endoplasmic reticulum. Biochem J. 1994 Jun 15;300(Pt 3):865–870. doi: 10.1042/bj3000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connern C. P., Halestrap A. P. Purification and N-terminal sequencing of peptidyl-prolyl cis-trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem J. 1992 Jun 1;284(Pt 2):381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesik S. W., Gampe R. T., Jr, Holzman T. F., Egan D. A., Edalji R., Luly J. R., Simmer R., Helfrich R., Kishore V., Rich D. H. Isotope-edited NMR of cyclosporin A bound to cyclophilin: evidence for a trans 9,10 amide bond. Science. 1990 Dec 7;250(4986):1406–1409. doi: 10.1126/science.2255910. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H., Mech C. Nachweis einer Enzymkatalyse für die cis-trans-Isomerisierung der Peptidbindung in prolinhaltigen Peptiden. Biomed Biochim Acta. 1984;43(10):1101–1111. [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry. 1990 Apr 24;29(16):3813–3816. doi: 10.1021/bi00468a001. [DOI] [PubMed] [Google Scholar]
- Hasel K. W., Glass J. R., Godbout M., Sutcliffe J. G. An endoplasmic reticulum-specific cyclophilin. Mol Cell Biol. 1991 Jul;11(7):3484–3491. doi: 10.1128/mcb.11.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U., Saenger W. Specific protein-nucleic acid recognition in ribonuclease T1-2'-guanylic acid complex: an X-ray study. Nature. 1982 Sep 2;299(5878):27–31. doi: 10.1038/299027a0. [DOI] [PubMed] [Google Scholar]
- Kallen J., Spitzfaden C., Zurini M. G., Wider G., Widmer H., Wüthrich K., Walkinshaw M. D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991 Sep 19;353(6341):276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zydowsky L. D., Liu J., Walsh C. T. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefhaber T., Grunert H. P., Hahn U., Schmid F. X. Replacement of a cis proline simplifies the mechanism of ribonuclease T1 folding. Biochemistry. 1990 Jul 10;29(27):6475–6480. doi: 10.1021/bi00479a020. [DOI] [PubMed] [Google Scholar]
- Kiefhaber T., Quaas R., Hahn U., Schmid F. X. Folding of ribonuclease T1. 1. Existence of multiple unfolded states created by proline isomerization. Biochemistry. 1990 Mar 27;29(12):3053–3061. doi: 10.1021/bi00464a023. [DOI] [PubMed] [Google Scholar]
- Kiefhaber T., Quaas R., Hahn U., Schmid F. X. Folding of ribonuclease T1. 2. Kinetic models for the folding and unfolding reactions. Biochemistry. 1990 Mar 27;29(12):3061–3070. doi: 10.1021/bi00464a024. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michnick S. W., Rosen M. K., Wandless T. J., Karplus M., Schreiber S. L. Solution structure of FKBP, a rotamase enzyme and receptor for FK506 and rapamycin. Science. 1991 May 10;252(5007):836–839. doi: 10.1126/science.1709301. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Schönbrunner E. R., Mayer S., Tropschug M., Fischer G., Takahashi N., Schmid F. X. Catalysis of protein folding by cyclophilins from different species. J Biol Chem. 1991 Feb 25;266(6):3630–3635. [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Spik G., Haendler B., Delmas O., Mariller C., Chamoux M., Maes P., Tartar A., Montreuil J., Stedman K., Kocher H. P. A novel secreted cyclophilin-like protein (SCYLP). J Biol Chem. 1991 Jun 15;266(17):10735–10738. [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tangen O., Jonsson J., Orrenius S. Isolation of rat liver microsomes by gel filtration. Anal Biochem. 1973 Aug;54(2):597–603. doi: 10.1016/0003-2697(73)90392-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Rayner D., Baines A. J. Identification and partial purification of ABGP205, an integral membrane glycoprotein from brain that binds ankyrin. Biochem J. 1988 Jul 15;253(2):345–350. doi: 10.1042/bj2530345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science. 1991 May 10;252(5007):839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]