Abstract
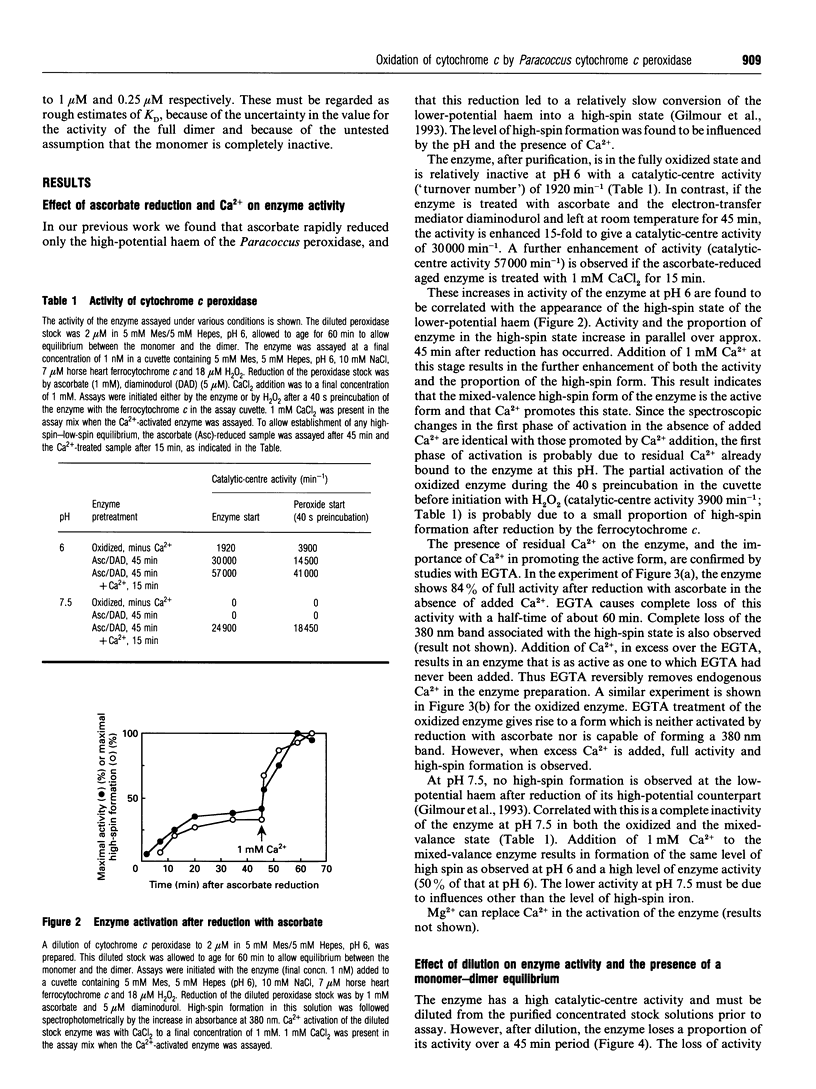
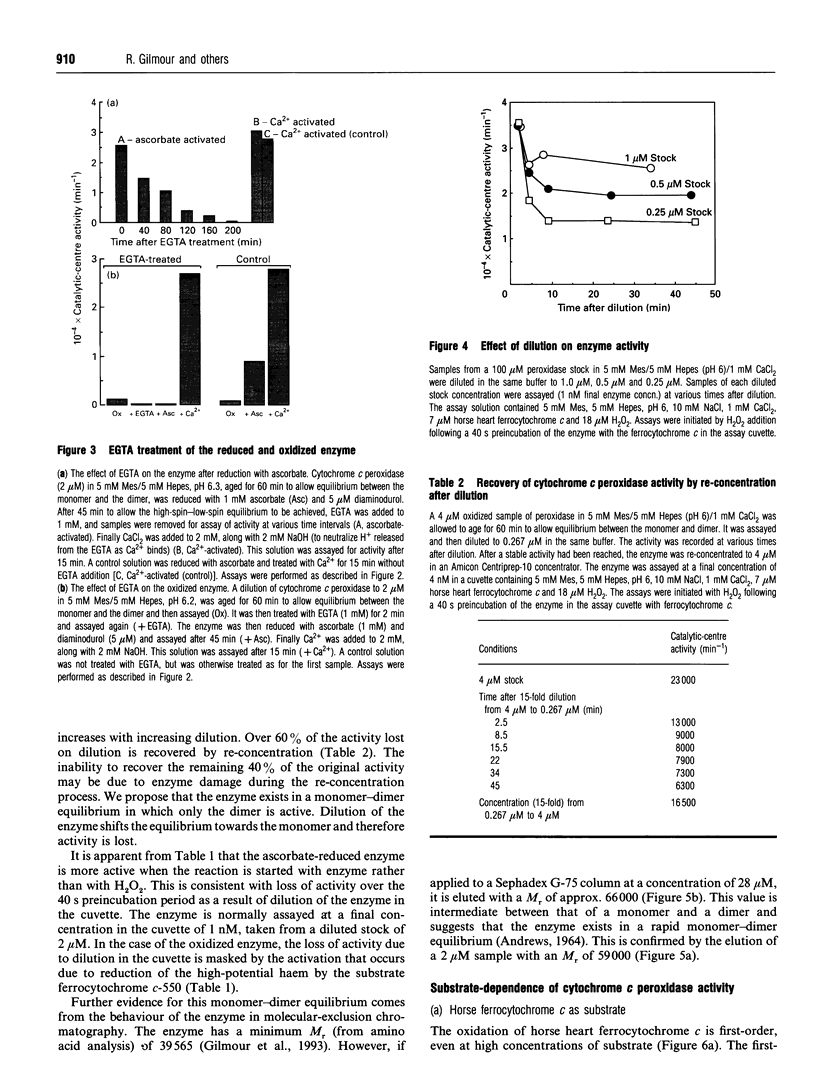
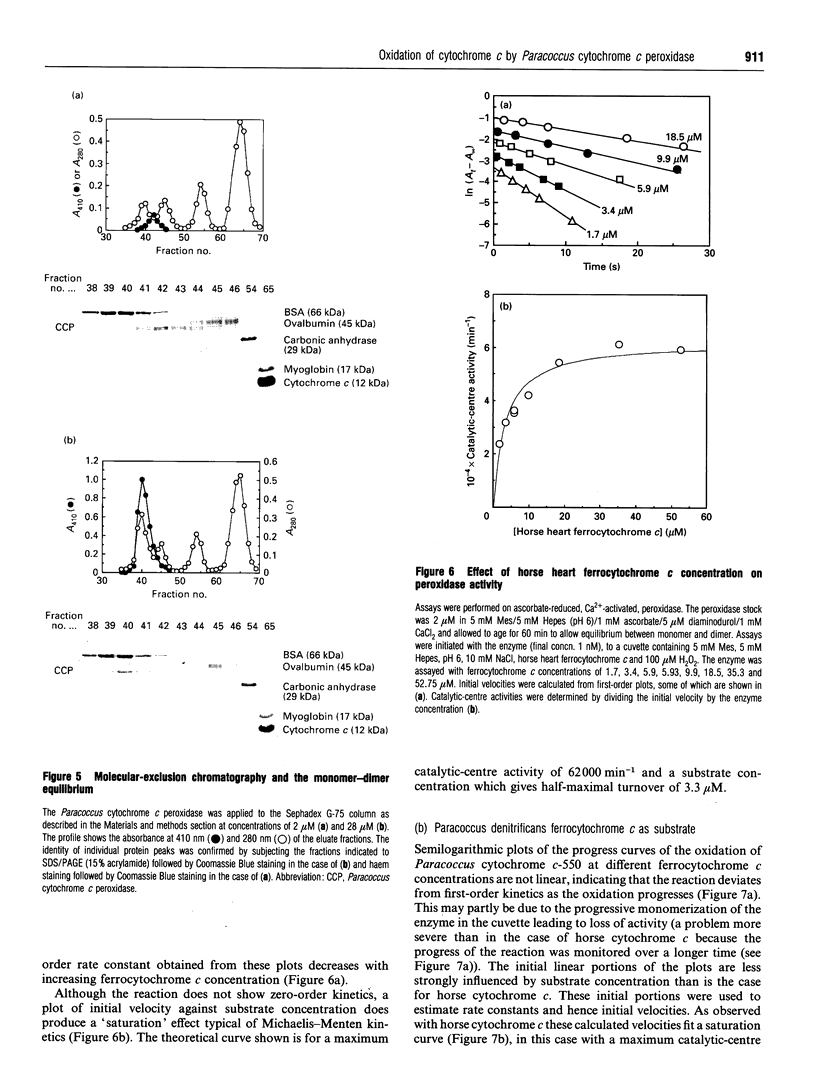
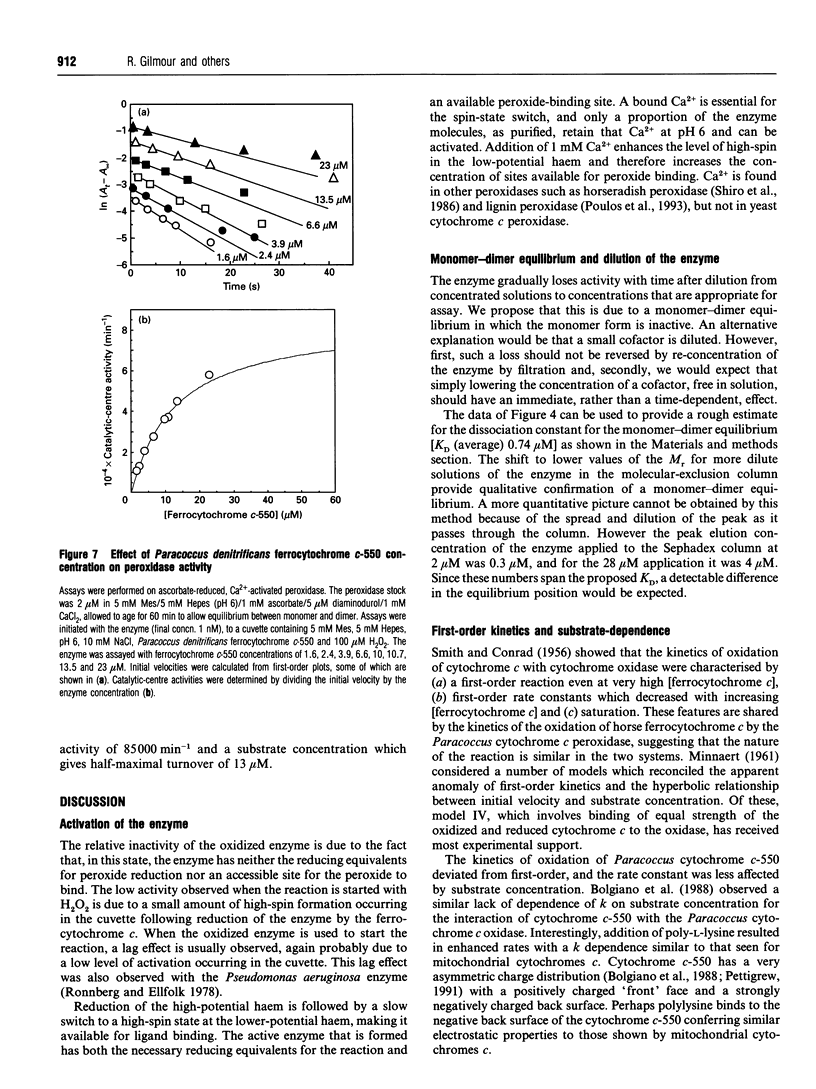
In work that is complementary to our investigation of the spectroscopic features of the cytochrome c peroxidase from Paracoccus denitrificans [Gilmour, Goodhew, Pettigrew, Prazeres, Moura and Moura (1993) Biochem. J. 294, 745-752], we have studied the kinetics of oxidation of cytochrome c by this enzyme. The enzyme, as isolated, is in the fully oxidized form and is relatively inactive. Reduction of the high-potential haem at pH 6 with ascorbate results in partial activation of the enzyme. Full activation is achieved by addition of 1 mM CaCl2. Enzyme activation is associated with formation of a high-spin state at the oxidized low-potential haem. EGTA treatment of the oxidized enzyme prevents activation after reduction with ascorbate, while treatment with EGTA of the reduced, partially activated, form abolishes the activity. We conclude that the active enzyme is a mixed-valence form with the low-potential haem in a high-spin state that is stabilized by Ca2+. Dilution of the enzyme results in a progressive loss of activity, the extent of which depends on the degree of dilution. Most of the activity lost upon dilution can be recovered after reconcentration. The M(r) of the enzyme on molecular-exclusion chromatography is concentration-dependent, with a shift to lower values at lower concentrations. Values of M(r) obtained are intermediate between those of a monomer (39,565) and a dimer. We propose that the active form of the enzyme is a dimer which dissociates at high dilution to give inactive monomers. From the activity of the enzyme at different dilutions, a KD of 0.8 microM can be calculated for the monomerdimer equilibrium. The cytochrome c peroxidase oxidizes horse ferrocytochrome c with first-order kinetics, even at high ferrocytochrome c concentrations. The maximal catalytic-centre activity ('turnover number') under the assay conditions used is 62,000 min-1, with a half-saturating ferrocytochrome c concentration of 3.3 microM. The corresponding values for the Paracoccus cytochrome c-550 (presumed to be the physiological substrate) are 85,000 min-1 and 13 microM. However, in this case, the kinetics deviate from first-order progress curves at all ferrocytochrome c concentrations. Consideration of the periplasmic environment in Paracoccus denitrificans leads us to propose that the enzyme will be present as the fully active dimer supplied with saturating ferrocytochrome c-550.
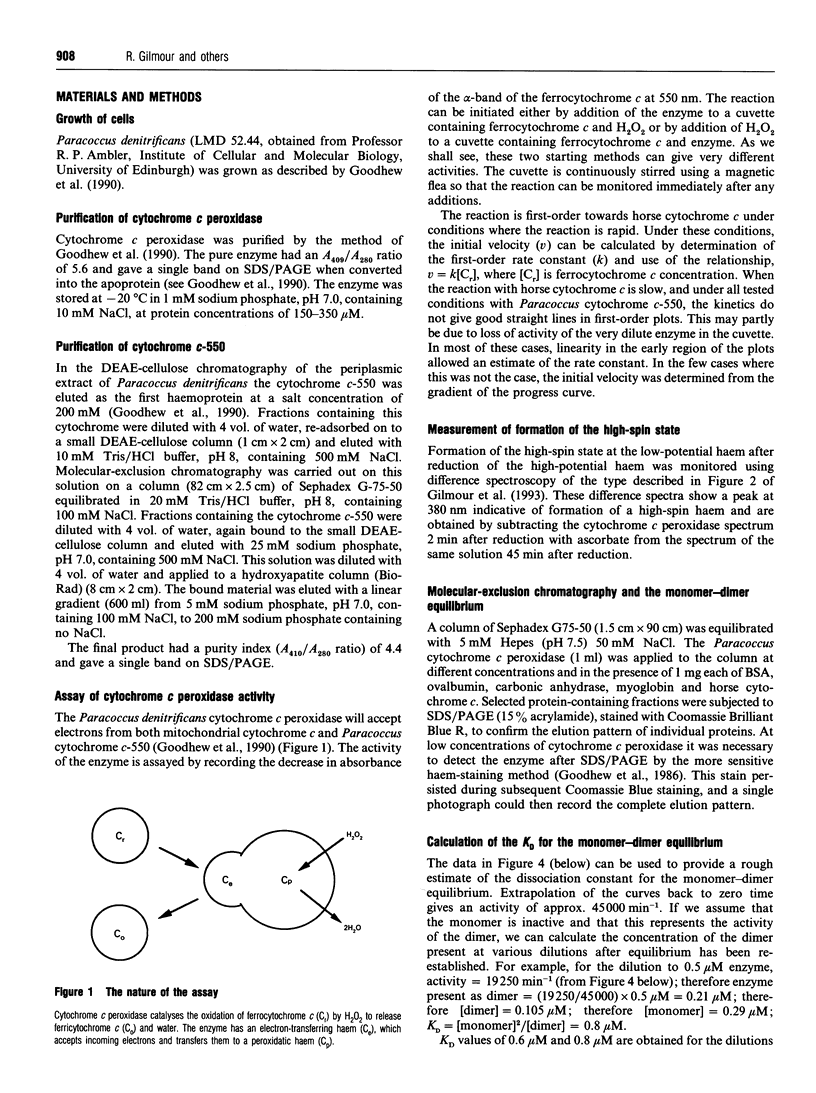
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini E., Brunori M., Colosimo A., Greenwood C., Wilson M. T. Oxygen "pulsed" cytochrome c oxidase: functional properties and catalytic relevance. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3128–3132. doi: 10.1073/pnas.74.8.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araiso T., Rönnberg M., Dunford H. B., Ellfolk N. The formation of the primary compound from hydrogen peroxide and Pseudomonas cytochrome c peroxidase. FEBS Lett. 1980 Aug 25;118(1):99–102. doi: 10.1016/0014-5793(80)81227-0. [DOI] [PubMed] [Google Scholar]
- Bolgiano B., Smith L., Davies H. C. Kinetics of the interaction of the cytochrome c oxidase of Paracoccus denitrificans with its own and bovine cytochrome c. Biochim Biophys Acta. 1988 Apr 22;933(2):341–350. doi: 10.1016/0005-2728(88)90041-2. [DOI] [PubMed] [Google Scholar]
- CONRAD H., SMITH L. A study of the kinetics of the oxidation of cytochrome c by cytochrome c oxidase. Arch Biochem Biophys. 1956 Aug;63(2):403–413. doi: 10.1016/0003-9861(56)90055-8. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denariaz C. M., Liu M. Y., Payne W. J., LeGall J., Marquez L., Dunford H. B., Van Beeumen J. Cytochrome c peroxidase activity of a protease-modified form of cytochrome c-552 from the denitrifying bacterium Pseudomonas perfectomarina. Arch Biochem Biophys. 1989 Apr;270(1):114–125. doi: 10.1016/0003-9861(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Rönnberg M., Aasa R., Andréasson L. E., Vänngård T. Anion binding to resting and half-reduced Pseudomonas cytochrome c peroxidase. Biochim Biophys Acta. 1984 Jan 18;784(1):62–67. doi: 10.1016/0167-4838(84)90173-0. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Rönnberg M., Aasa R., Andréasson L. E., Vänngård T. Properties and function of the two hemes in Pseudomonas cytochrome c peroxidase. Biochim Biophys Acta. 1983 Feb 28;743(1):23–30. doi: 10.1016/0167-4838(83)90413-2. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Soininen R. Pseudomonas cytochrome c peroxidase. I. Purification procedure. Acta Chem Scand. 1970;24(6):2126–2136. doi: 10.3891/acta.chem.scand.24-2126. [DOI] [PubMed] [Google Scholar]
- Foote N., Peterson J., Gadsby P. M., Greenwood C., Thomson A. J. A study of the oxidized form of Pseudomonas aeruginosa cytochrome c-551 peroxidase with the use of magnetic circular dichroism. Biochem J. 1984 Oct 15;223(2):369–378. doi: 10.1042/bj2230369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote N., Thompson A. C., Barber D., Greenwood C. Pseudomonas cytochrome C-551 peroxidase. A purification procedure and study of CO-binding kinetics. Biochem J. 1983 Mar 1;209(3):701–707. doi: 10.1042/bj2090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R., Goodhew C. F., Pettigrew G. W., Prazeres S., Moura I., Moura J. J. Spectroscopic characterization of cytochrome c peroxidase from Paracoccus denitrificans. Biochem J. 1993 Sep 15;294(Pt 3):745–752. doi: 10.1042/bj2940745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhew C. F., Wilson I. B., Hunter D. J., Pettigrew G. W. The cellular location and specificity of bacterial cytochrome c peroxidases. Biochem J. 1990 Nov 1;271(3):707–712. doi: 10.1042/bj2710707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- MINNAERT K. The kinetics of cytochrome c oxidase. I. The system: cytochrome c-cytochrome oxidase-oxygen. Biochim Biophys Acta. 1961 Jun 10;50:23–34. doi: 10.1016/0006-3002(61)91055-1. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The cytochrome c peroxidase of Paracoccus denitrificans. Biochim Biophys Acta. 1991 May 23;1058(1):25–27. doi: 10.1016/s0005-2728(05)80261-0. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Edwards S. L., Wariishi H., Gold M. H. Crystallographic refinement of lignin peroxidase at 2 A. J Biol Chem. 1993 Feb 25;268(6):4429–4440. doi: 10.2210/pdb1lga/pdb. [DOI] [PubMed] [Google Scholar]
- Rönnberg M., Ellfolk N. Pseudomonas cytochrome c peroxidase. Initial delay of the peroxidatic reaction. Electron transfer properties. Biochim Biophys Acta. 1978 Oct 11;504(1):60–66. doi: 10.1016/0005-2728(78)90006-3. [DOI] [PubMed] [Google Scholar]
- Shiro Y., Kurono M., Morishima I. Presence of endogenous calcium ion and its functional and structural regulation in horseradish peroxidase. J Biol Chem. 1986 Jul 15;261(20):9382–9390. [PubMed] [Google Scholar]
- Soininen R., Ellfolk N. Pseudomonas cytochrome c peroxidase. IV. Some kinetic properties of the peroxidation reaction, and enzymatic determination of the extinction coefficients of Pseudomonas cytochrome c-551 and azurin. Acta Chem Scand. 1972;26(3):861–872. doi: 10.3891/acta.chem.scand.26-0861. [DOI] [PubMed] [Google Scholar]
- Soininen R., Sojonen H., Ellfolk N. Pseudomonas cytochrome c peroxidase. II. Localization of cytochrome c peroxidase in Pseudomonas fluorescens. Acta Chem Scand. 1970;24(7):2314–2320. doi: 10.3891/acta.chem.scand.24-2314. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Villalaín J., Moura I., Liu M. C., Payne W. J., LeGall J., Xavier A. V., Moura J. J. NMR and electron-paramagnetic-resonance studies of a dihaem cytochrome from Pseudomonas stutzeri (ATCC 11607) (cytochrome c peroxidase). Eur J Biochem. 1984 Jun 1;141(2):305–312. doi: 10.1111/j.1432-1033.1984.tb08192.x. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. 3. Kinetics of the peroxidatic oxidation of ferrocytochrome c catalyzed by cytochrome c peroxidase. J Biol Chem. 1966 Feb 10;241(3):700–706. [PubMed] [Google Scholar]