Abstract
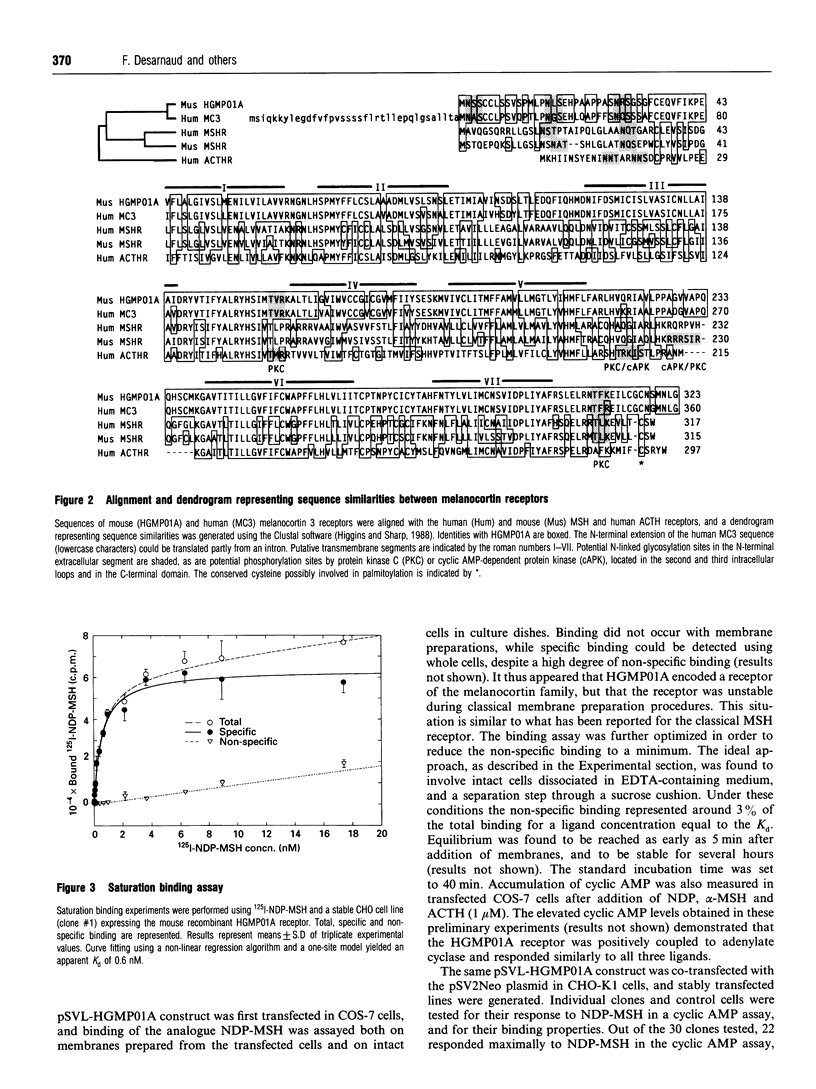
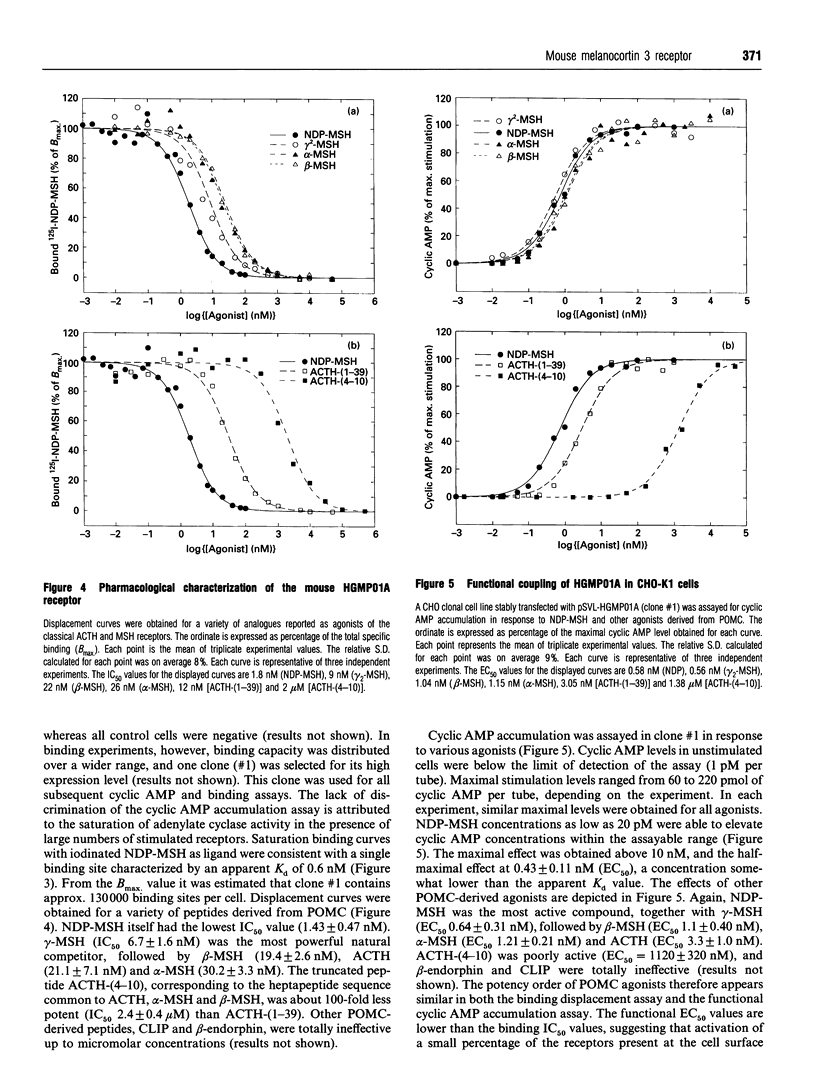
We describe the cloning of the mouse HGMP01A gene that encodes a melanocortin receptor functionally distinct from the adrenal cortex corticotropin (adrenocorticotrophic hormone; ACTH) receptor and the melanocyte-stimulating hormone (MSH) receptor expressed in melanoma. The gene encodes a protein of 323 amino acids with a calculated molecular mass of 35,800 Da, displaying potential sites for N-linked glycosylation and phosphorylation by protein kinase C. An RNAase protection assay detected weak expression in the brain, but not in adrenal gland, skin, or any of the other tissues tested. Stable CHO cell lines expressing over 100,000 receptors per cell were generated. The recombinant receptor binds iodinated [Nle4,D-Phe7]alpha-MSH (NDP-MSH) with an apparent Kd of 700 pM. Displacement of the ligand by a variety of pro-opiomelanocortin-derived peptides revealed a pharmacological profile distinct from that of the classical ACTH and MSH receptors. NDP-MSH was the most powerful competitor (IC50 1.4 nM), followed by gamma-MSH (IC50 7 nM). alpha-MSH, beta-MSH and ACTH-(1-39) were significantly less potent, with IC50 values of 30, 19 and 21 nM respectively. ACTH-(4-10) was poorly active (IC50 2.4 microM), while corticotropin-like intermediate lobe peptide (CLIP) and beta-endorphin were totally ineffective. The recombinant receptor was found to stimulate adenylate cyclase. The potency order of the agonists in this assay was consistent with that of the binding displacement assays. This receptor represents the orthologue of the human melanocortin 3 receptor reported recently. The growing family of melanocortin receptors constitute the molecular basis for the variety of actions of melanocortins that have been described over the years. The availability of functionally expressed receptors from the melanocortin family will allow the development of a specific pharmacology, and a better understanding of the function of the pro-opiomelanocortin-derived peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C. L., Chang C. C., Krieger D. T., Bardin C. W. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology. 1986 Jun;118(6):2382–2389. doi: 10.1210/endo-118-6-2382. [DOI] [PubMed] [Google Scholar]
- Chhajlani V., Wikberg J. E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992 Sep 14;309(3):417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- De Wied D., Croiset G. Stress modulation of learning and memory processes. Methods Achiev Exp Pathol. 1991;15:167–199. [PubMed] [Google Scholar]
- De Wied D., Jolles J. Neuropeptides derived from pro-opiocortin: behavioral, physiological, and neurochemical effects. Physiol Rev. 1982 Jul;62(3):976–1059. doi: 10.1152/physrev.1982.62.3.976. [DOI] [PubMed] [Google Scholar]
- DeBold C. R., Menefee J. K., Nicholson W. E., Orth D. N. Proopiomelanocortin gene is expressed in many normal human tissues and in tumors not associated with ectopic adrenocorticotropin syndrome. Mol Endocrinol. 1988 Sep;2(9):862–870. doi: 10.1210/mend-2-9-862. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Ehymayed H. M., Janský L. A discrete mode of the antipyretic action of AVP, alpha-MSH and ACTH. Physiol Res. 1992;41(1):57–61. [PubMed] [Google Scholar]
- Gantz I., Konda Y., Tashiro T., Shimoto Y., Miwa H., Munzert G., Watson S. J., DelValle J., Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993 Apr 15;268(11):8246–8250. [PubMed] [Google Scholar]
- Gruber K. A., Callahan M. F. ACTH-(4-10) through gamma-MSH: evidence for a new class of central autonomic nervous system-regulating peptides. Am J Physiol. 1989 Oct;257(4 Pt 2):R681–R694. doi: 10.1152/ajpregu.1989.257.4.R681. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hiltz M. E., Catania A., Lipton J. M. Alpha-MSH peptides inhibit acute inflammation induced in mice by rIL-1 beta, rIL-6, rTNF-alpha and endogenous pyrogen but not that caused by LTB4, PAF and rIL-8. Cytokine. 1992 Jul;4(4):320–328. doi: 10.1016/1043-4666(92)90073-z. [DOI] [PubMed] [Google Scholar]
- Hiltz M. E., Catania A., Lipton J. M. Anti-inflammatory activity of alpha-MSH(11-13) analogs: influences of alteration in stereochemistry. Peptides. 1991 Jul-Aug;12(4):767–771. doi: 10.1016/0196-9781(91)90131-8. [DOI] [PubMed] [Google Scholar]
- Jingami H., Nakanishi S., Imura H., Numa S. Tissue distribution of messenger RNAs coding for opioid peptide precursors and related RNA. Eur J Biochem. 1984 Aug 1;142(3):441–447. doi: 10.1111/j.1432-1033.1984.tb08306.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Lipton J. M., Macaluso A., Hiltz M. E., Catania A. Central administration of the peptide alpha-MSH inhibits inflammation in the skin. Peptides. 1991 Jul-Aug;12(4):795–798. doi: 10.1016/0196-9781(91)90135-c. [DOI] [PubMed] [Google Scholar]
- McCaig C. D., Stewart R. The effects of melanocortins and electrical fields on neuronal growth. Exp Neurol. 1992 May;116(2):172–179. doi: 10.1016/0014-4886(92)90165-m. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy K. G., Robbins L. S., Mortrud M. T., Cone R. D. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992 Aug 28;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- Parmentier M., Libert F., Maenhaut C., Lefort A., Gérard C., Perret J., Van Sande J., Dumont J. E., Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989 Dec 22;246(4937):1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- Perret J., Ludgate M., Libert F., Gerard C., Dumont J. E., Vassart G., Parmentier M. Stable expression of the human TSH receptor in CHO cells and characterization of differentially expressing clones. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1044–1050. doi: 10.1016/0006-291x(90)90789-p. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Robbins L. S., Nadeau J. H., Johnson K. R., Kelly M. A., Roselli-Rehfuss L., Baack E., Mountjoy K. G., Cone R. D. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993 Mar 26;72(6):827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Scott J. J. Recovery of denervated muscle receptors following treatments to accelerate nerve regeneration. Brain Res. 1991 Nov 1;563(1-2):195–202. doi: 10.1016/0006-8993(91)91533-7. [DOI] [PubMed] [Google Scholar]
- Siegrist W., Oestreicher M., Stutz S., Girard J., Eberle A. N. Radioreceptor assay for alpha-MSH using mouse B16 melanoma cells+. J Recept Res. 1988;8(1-4):323–343. doi: 10.3109/10799898809048996. [DOI] [PubMed] [Google Scholar]
- Strand F. L., Rose K. J., Zuccarelli L. A., Kume J., Alves S. E., Antonawich F. J., Garrett L. Y. Neuropeptide hormones as neurotrophic factors. Physiol Rev. 1991 Oct;71(4):1017–1046. doi: 10.1152/physrev.1991.71.4.1017. [DOI] [PubMed] [Google Scholar]
- Villar M., Perassi N., Celis M. E. Central and peripheral actions of alpha-MSH in the thermoregulation of rats. Peptides. 1991 Nov-Dec;12(6):1441–1443. doi: 10.1016/0196-9781(91)90231-d. [DOI] [PubMed] [Google Scholar]
- Weiss J. M., Sundar S. K., Cierpial M. A., Ritchie J. C. Effects of interleukin-1 infused into brain are antagonized by alpha-MSH in a dose-dependent manner. Eur J Pharmacol. 1991 Jan 3;192(1):177–179. doi: 10.1016/0014-2999(91)90087-7. [DOI] [PubMed] [Google Scholar]
- Zohar M., Salomon Y. Melanocortins stimulate proliferation and induce morphological changes in cultured rat astrocytes by distinct transducing mechanisms. Brain Res. 1992 Mar 27;576(1):49–58. doi: 10.1016/0006-8993(92)90608-c. [DOI] [PubMed] [Google Scholar]



