Abstract
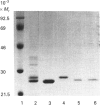
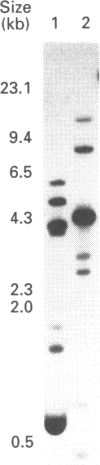
Three glutathione S-transferases from Lucilia cuprina (Australian sheep blowfly) pupae were purified by affinity chromatography and anion-exchange chromatography. One isoenzyme was composed of M(r)-24,800 subunits, and two isoenzymes had subunits of M(r) 23,900. The M(r)-23,900 subunits showed immunological identity and were immunologically distinct from the M(r)-24,800 subunits. All three enzymes were active with the substrate 1-chloro-2,4-dinitrobenzene and had low activity with 1,2-dichloro-4-nitrobenzene. A cDNA clone encoding a M(r)-23,900 subunit (LuGST1) was isolated and sequenced. The sequence has close similarities (> 81%) to that of GSTs from the fruitfly Drosophila melanogaster and Musca domestica (housefly). The deduced amino acid sequence of the Lu GST1 subunit showed no significant similarity to that of the mammalian GSTs to the Alpha, Mu and Pi classes, but shows some similarity (33%) over the first 100 residues with the rat subunit 12 Theta-class GST. Southern blots of genomic DNA hybridized with the LuGST1 cDNA identified many hybridizing fragments. Taken together, these data indicated that the L. cuprina genome contains multiple glutathione S-transferase genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Board P. G. Genetic heterogeneity of the B subunit of coagulation factor XIII: resolution of type 2. Ann Hum Genet. 1984 Jul;48(Pt 3):223–228. doi: 10.1111/j.1469-1809.1984.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Board P. G., Pierce K. Expression of human glutathione S-transferase 2 in Escherichia coli. Immunological comparison with the basic glutathione S-transferases isoenzymes from human liver. Biochem J. 1987 Dec 15;248(3):937–941. doi: 10.1042/bj2480937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P., Coggan M., Johnston P., Ross V., Suzuki T., Webb G. Genetic heterogeneity of the human glutathione transferases: a complex of gene families. Pharmacol Ther. 1990;48(3):357–369. doi: 10.1016/0163-7258(90)90054-6. [DOI] [PubMed] [Google Scholar]
- Brophy P. M., Southan C., Barrett J. Glutathione transferases in the tapeworm Moniezia expansa. Biochem J. 1989 Sep 15;262(3):939–946. doi: 10.1042/bj2620939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Marshall S. N., Qureshi A. R. Synthesis and use of an isoform-specific affinity matrix in the purification of glutathione S-transferases from the housefly, Musca domestica (L.). Protein Expr Purif. 1990 Nov;1(2):121–126. doi: 10.1016/1046-5928(90)90004-i. [DOI] [PubMed] [Google Scholar]
- Clark A. G. The comparative enzymology of the glutathione S-transferases from non-vertebrate organisms. Comp Biochem Physiol B. 1989;92(3):419–446. doi: 10.1016/0305-0491(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Motoyama N., Dauterman W. C. The role of nonoxidative metabolism in organophosphorus resistance. J Agric Food Chem. 1974 May-Jun;22(3):350–356. doi: 10.1021/jf60193a055. [DOI] [PubMed] [Google Scholar]
- Ogura K., Nishiyama T., Okada T., Kajital J., Narihata H., Watabe T., Hiratsuka A., Watabe T. Molecular cloning and amino acid sequencing of rat liver class theta glutathione S-transferase Yrs-Yrs inactivating reactive sulfate esters of carcinogenic arylmethanols. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1294–1300. doi: 10.1016/0006-291x(91)92079-y. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Toung Y. P., Tu C. P. Drosophila glutathione S-transferases have sequence homology to the stringent starvation protein of Escherichia coli. Biochem Biophys Res Commun. 1992 Jan 15;182(1):355–360. doi: 10.1016/s0006-291x(05)80152-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida S., Sato K. Glutathione transferases and cancer. Crit Rev Biochem Mol Biol. 1992;27(4-5):337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., McCommas S., Syvanen M. Molecular cloning of a glutathione S-transferase overproduced in an insecticide-resistant strain of the housefly (Musca domestica). Mol Gen Genet. 1991 Jun;227(2):260–266. doi: 10.1007/BF00259679. [DOI] [PubMed] [Google Scholar]