Abstract
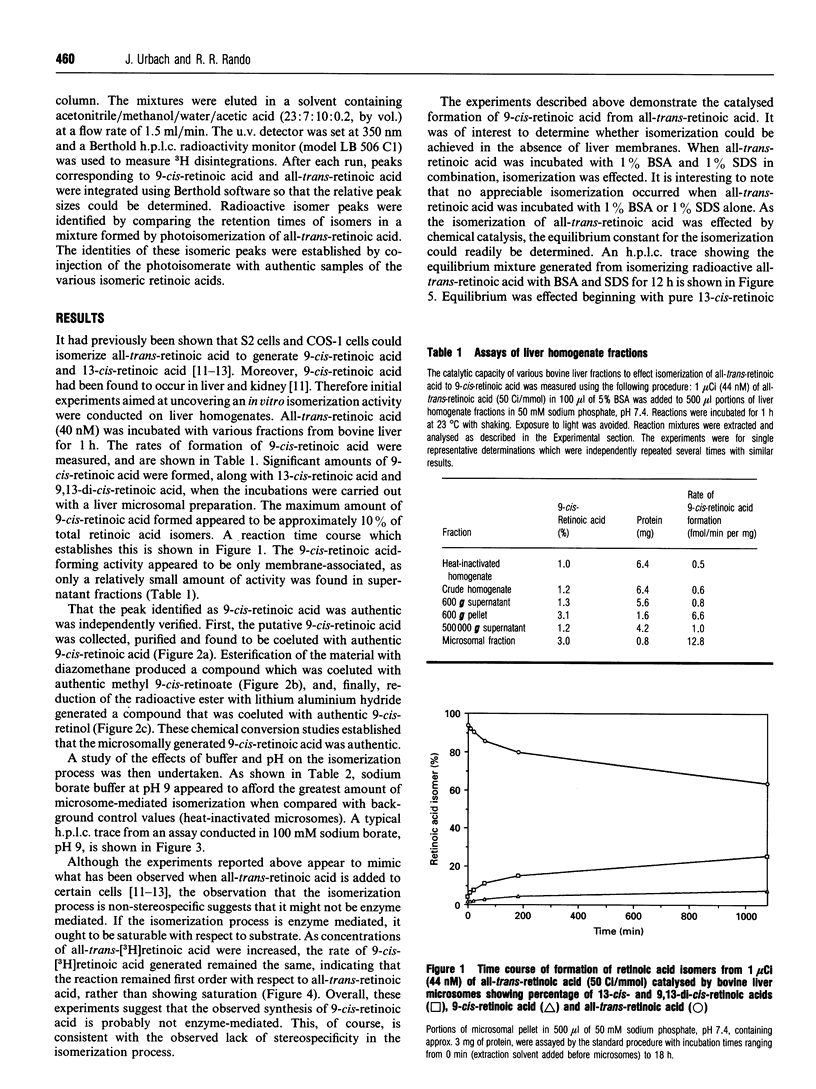
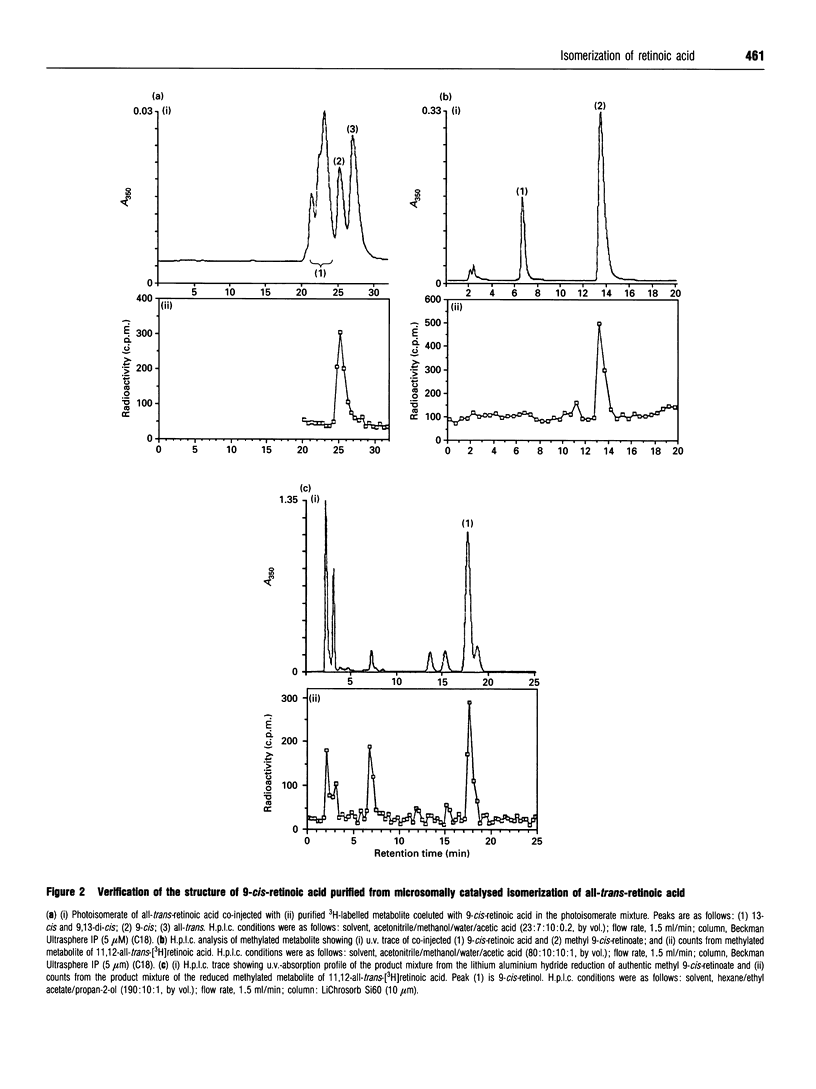
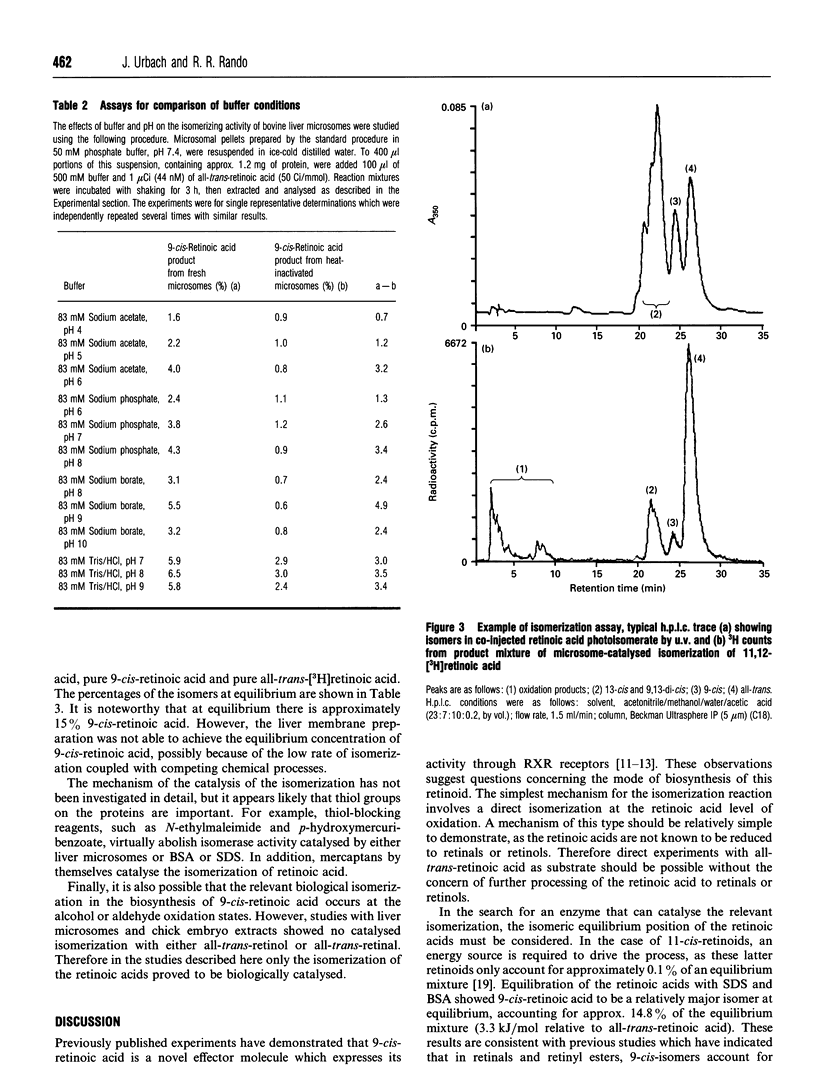
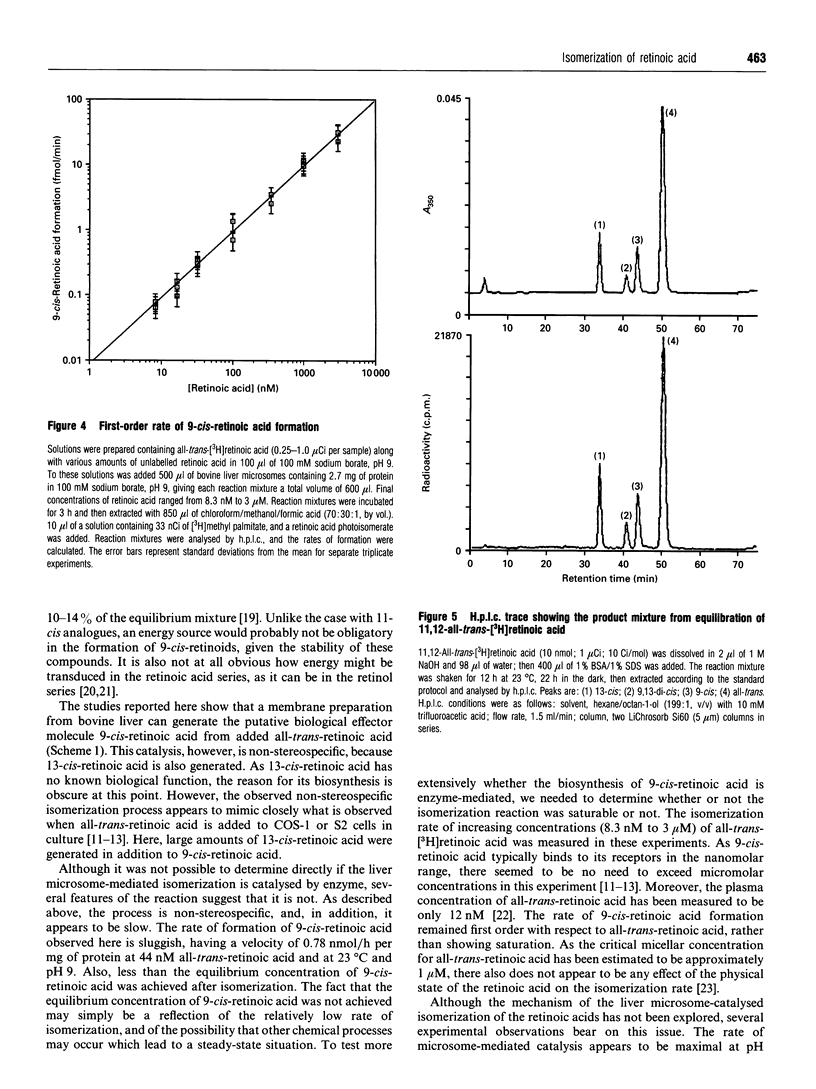
The discovery of the biological activity of 9-cis-retinoic acid raises questions as to its mode of biosynthesis. A simple mechanism involves the direct isomerization of all-trans-retinoic acid to 9-cis-retinoic acid. It is shown here that bovine liver membranes, but not supernatant fractions, can isomerize all-trans-retinoic acid into 9-cis-retinoic acid and 13-cis-retinoic acid. The concentration of 9-cis-retinoic acid generated approaches its equilibrium concentration, which is determined here to be approximately 15%. However, the isomerization process could not be shown to be saturable, and is first-order in all-trans-retinoic acid in the concentration range measured (8.3 nM to 3 microM). Isomerization reactions measured using bovine liver microsomes appear to be mediated by thiol groups, as they can be blocked by group-specific thiol-blocking reagents such as N-ethylmaleimide. It is interesting to note that the non-stereospecific behaviour observed here mimics what is observed when all-trans-retinoic acid is applied to cells. Finally, significant formation of 9-cis-retinoids was not found when the reaction was carried out with liver microsomes and either all-trans-retinol or all-trans-retinal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Mokady S., Avron M. The beta-carotene-rich alga Dunaliella bardawil as a source of retinol in a rat diet. Br J Nutr. 1988 May;59(3):443–449. doi: 10.1079/bjn19880053. [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A., Mokady S., Edelstein S., Avron M. Bioavailability of a natural isomer mixture as compared with synthetic all-trans-beta-carotene in rats and chicks. J Nutr. 1989 Jul;119(7):1013–1019. doi: 10.1093/jn/119.7.1013. [DOI] [PubMed] [Google Scholar]
- Bernstein P. S., Law W. C., Rando R. R. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein P. S., Rando R. R. In vivo isomerization of all-trans- to 11-cis-retinoids in the eye occurs at the alcohol oxidation state. Biochemistry. 1986 Oct 21;25(21):6473–6478. doi: 10.1021/bi00369a020. [DOI] [PubMed] [Google Scholar]
- Brockes J. Developmental biology. Reading the retinoid signals. Nature. 1990 Jun 28;345(6278):766–768. doi: 10.1038/345766a0. [DOI] [PubMed] [Google Scholar]
- De Leenheer A. P., Lambert W. E., Claeys I. All-trans-retinoic acid: measurement of reference values in human serum by high performance liquid chromatography. J Lipid Res. 1982 Dec;23(9):1362–1367. [PubMed] [Google Scholar]
- Durand B., Saunders M., Leroy P., Leid M., Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992 Oct 2;71(1):73–85. doi: 10.1016/0092-8674(92)90267-g. [DOI] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M., Jong L., Fanjul A., Cameron J. F., Lu X. P., Haefner P., Dawson M. I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992 Dec 18;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Matsushima Y., Kawachi E., Tanaka H., Kagechika H., Hashimoto Y., Shudo K. Differentiation-inducing activity of retinoic acid isomers and their oxidized analogs on human promyelocytic leukemia HL-60 cells. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1136–1142. doi: 10.1016/0006-291x(92)92322-o. [DOI] [PubMed] [Google Scholar]
- Noy N. The ionization behavior of retinoic acid in aqueous environments and bound to serum albumin. Biochim Biophys Acta. 1992 Apr 29;1106(1):151–158. doi: 10.1016/0005-2736(92)90233-c. [DOI] [PubMed] [Google Scholar]
- Ollilainen V., Heinonen M., Linkola E., Varo P., Koivistoinen P. Carotenoids and retinoids in Finnish foods: dairy products and eggs. J Dairy Sci. 1989 Sep;72(9):2257–2265. doi: 10.3168/jds.S0022-0302(89)79356-5. [DOI] [PubMed] [Google Scholar]
- Posch K. C., Burns R. D., Napoli J. L. Biosynthesis of all-trans-retinoic acid from retinal. Recognition of retinal bound to cellular retinol binding protein (type I) as substrate by a purified cytosolic dehydrogenase. J Biol Chem. 1992 Sep 25;267(27):19676–19682. [PubMed] [Google Scholar]
- Rando R. R. Membrane phospholipids and the dark side of vision. J Bioenerg Biomembr. 1991 Feb;23(1):133–146. doi: 10.1007/BF00768843. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Membrane phospholipids as an energy source in the operation of the visual cycle. Biochemistry. 1991 Jan 22;30(3):595–602. doi: 10.1021/bi00217a001. [DOI] [PubMed] [Google Scholar]
- SWORN B. R. Total cystectomy. Br Med J. 1949 Feb 5;1(4596):221–223. doi: 10.1136/bmj.1.4596.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W., Schwarz W., Sundquist A. R., Sies H. cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem Biophys. 1992 Apr;294(1):173–177. doi: 10.1016/0003-9861(92)90153-n. [DOI] [PubMed] [Google Scholar]
- Summerbell D., Maden M. Retinoic acid, a developmental signalling molecule. Trends Neurosci. 1990 Apr;13(4):142–147. doi: 10.1016/0166-2236(90)90006-v. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Isolation of 3,4-didehydroretinoic acid, a novel morphogenetic signal in the chick wing bud. Nature. 1990 Jun 28;345(6278):815–819. doi: 10.1038/345815a0. [DOI] [PubMed] [Google Scholar]


