Abstract
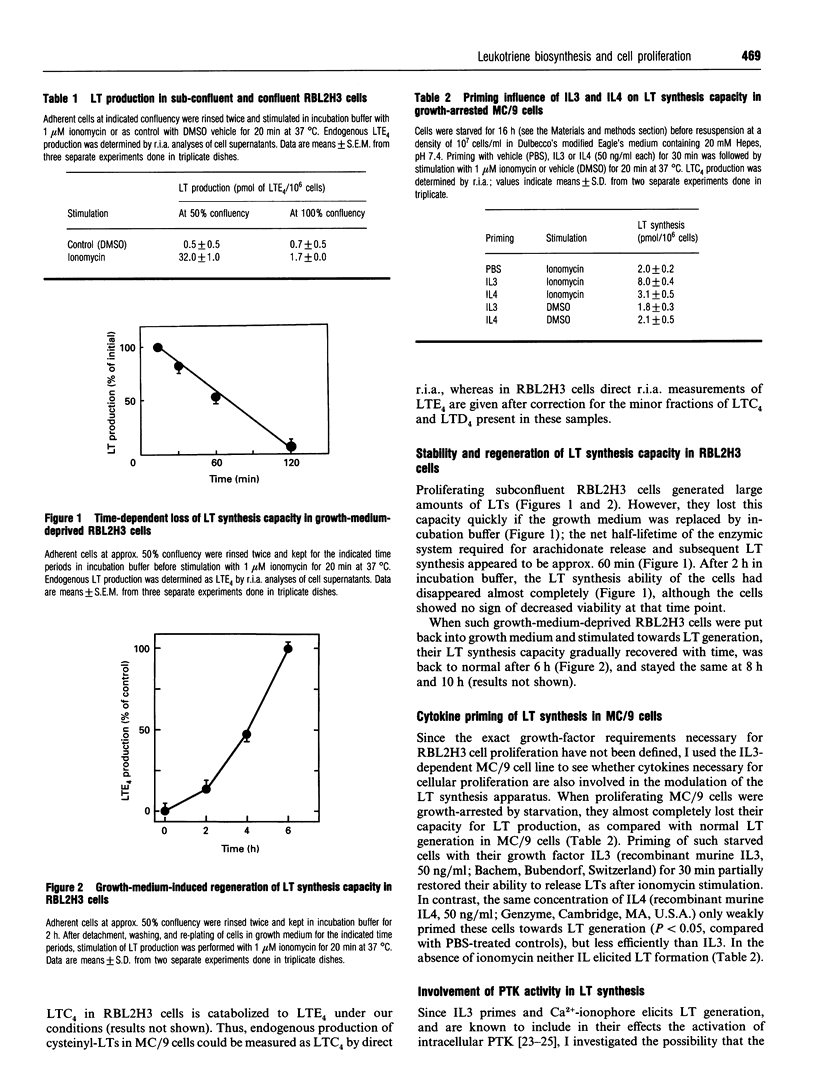
Mast cells, mastocytoma cells and basophil leukaemia cells are well-established producers of leukotrienes when grown and stimulated appropriately. I report that the cells' ability to produce leukotrienes is dependent on the cells' proliferative status or their provision with growth factors. Proliferating MC/9 and subconfluent RBL2H3 cells respond maximally to stimulation by 1 microM ionomycin with the production of 56 and 32 pmol of cysteinyl-leukotrienes/10(6) cells respectively. In contrast, confluent RBL2H3 or growth-arrested MC/9 cells lose their ability to generate leukotrienes in response to ionomycin treatment. This rapid down-regulation of leukotriene synthesis is also observed when proliferating RBL2H3 cells are transferred to growth-factor-free medium, wherein cellular leukotriene-synthesis capacity has an apparent half-lifetime of 60 min. Transfer back into growth medium results in the regeneration of leukotriene synthesis capacity within 6 h. In growth-arrested MC/9 cells, leukotriene production ability can at least partially be restored by priming the cells with interleukin 3, but not with interleukin 4. In RBL2H3 cells, pretreatment with protein tyrosine kinase inhibitors such as genistein (5 min, 37 microM), herbimycin A (6 h, 3 microM) or tyrphostin 25 (16 h, 100 microM) completely inhibits leukotriene generation, whereas okadaic acid (15 min, 0.5 microM) has no effect. Under these conditions, both genistein and herbimycin A strongly impair ionomycin-induced protein tyrosine phosphorylation. Our study indicates that leukotriene generation in these tumour cells is tightly regulated by their proliferation status and supply with growth factors, and cell stimulation towards leukotriene synthesis appears to involve protein tyrosine kinase activity.
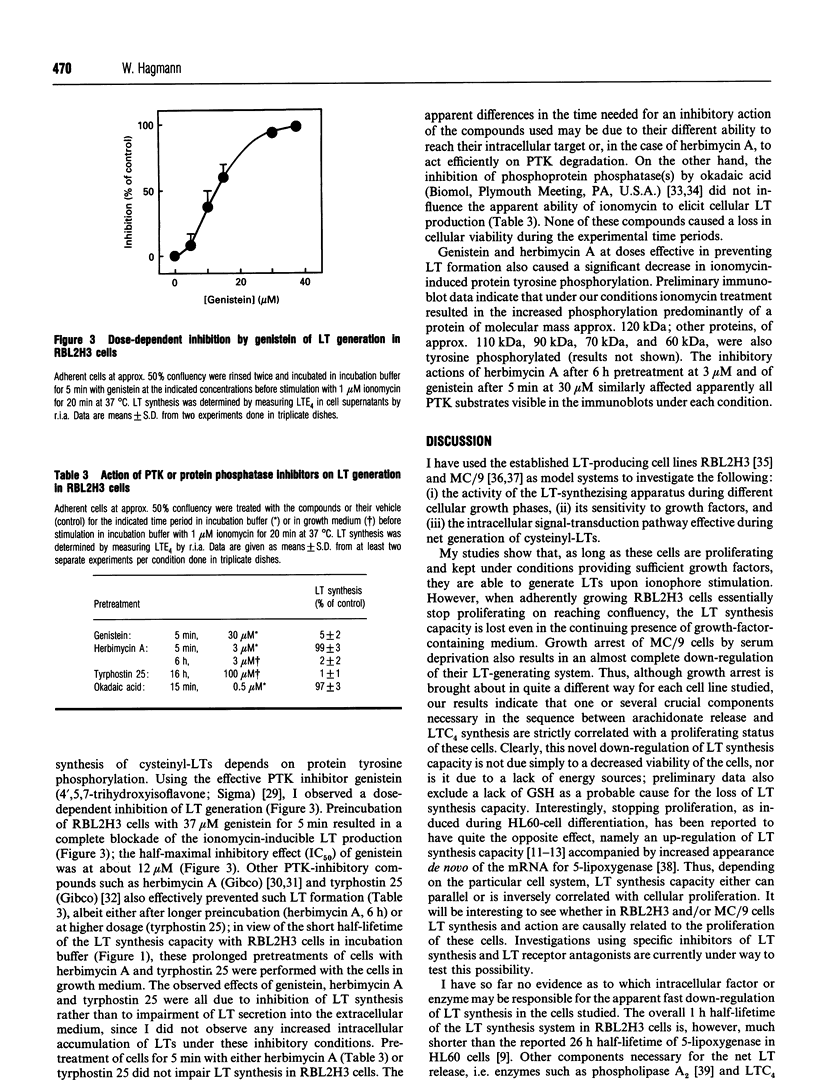
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Anthes J. C., Bryant R. W., Musch M. W., Ng K., Siegel M. I. Calcium ionophore and chemotactic peptide stimulation of peptidoleukotriene synthesis in DMSO-differentiated HL60 cells. Inflammation. 1986 Jun;10(2):145–156. doi: 10.1007/BF00915996. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S. C., Baggiolini M., de Weck A. L., Dahinden C. A. Interleukin 8-inhibitor and inducer of histamine and leukotriene release in human basophils. Biochem Biophys Res Commun. 1991 Aug 30;179(1):628–633. doi: 10.1016/0006-291x(91)91418-c. [DOI] [PubMed] [Google Scholar]
- Bischoff S. C., Brunner T., De Weck A. L., Dahinden C. A. Interleukin 5 modifies histamine release and leukotriene generation by human basophils in response to diverse agonists. J Exp Med. 1990 Dec 1;172(6):1577–1582. doi: 10.1084/jem.172.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser R. W., Siegel M. I., McConnell R. T., Cuatrecasas P. Chemotactic peptide stimulated endogenous arachidonic acid metabolism in HL-60 granulocytes. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1269–1275. doi: 10.1016/s0006-291x(81)80148-9. [DOI] [PubMed] [Google Scholar]
- Chaikin E., Ziltener H. J., Razin E. Protein kinase C plays an inhibitory role in interleukin 3- and interleukin 4-mediated mast cell proliferation. J Biol Chem. 1990 Dec 25;265(36):22109–22116. [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Milona N., Knopf J. L. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Chen M. J., Crooke S. T., Bomalaski J. S. Tumour necrosis factor (cachectin) induces phospholipase A2 activity and synthesis of a phospholipase A2-activating protein in endothelial cells. Biochem J. 1988 Feb 15;250(1):125–132. doi: 10.1042/bj2500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Kurimoto Y., De Weck A. L., Lindley I., Dewald B., Baggiolini M. The neutrophil-activating peptide NAF/NAP-1 induces histamine and leukotriene release by interleukin 3-primed basophils. J Exp Med. 1989 Nov 1;170(5):1787–1792. doi: 10.1084/jem.170.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Zingg J., Maly F. E., de Weck A. L. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988 Apr 1;167(4):1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Jones R. E., Diehl R. E., Bennett C. D., Kargman S., Rouzer C. A. Cloning of the cDNA for human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):416–420. doi: 10.1073/pnas.85.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa H., Li P. M., Yamamoto C., Murakami Y., Mizuno S., Uehara Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem Pharmacol. 1991 Oct 9;42(9):1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Gilfillan A. M., Wiggan G. A., Welton A. F. Pertussis toxin pretreatment reveals differential effects of adenosine analogs on IgE-dependent histamine and peptidoleukotriene release from RBL-2H3 cells. Biochim Biophys Acta. 1990 May 22;1052(3):467–474. doi: 10.1016/0167-4889(90)90157-9. [DOI] [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Zeh W., Salbach P., Kommerell B., Rothe D. E., Nastainczyk W., Glomset J. A. Evidence for coordinate, selective regulation of eicosanoid synthesis in platelet-derived growth factor-stimulated 3T3 fibroblasts and in HL-60 cells induced to differentiate into macrophages or neutrophils. J Biol Chem. 1988 Dec 25;263(36):19384–19391. [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Rothe D. E., Specht E., Ziegler R., Glomset J. A., Graf T. Early reversible induction of leukotriene synthesis in chicken myelomonocytic cells transformed by a temperature-sensitive mutant of avian leukemia virus E26. Proc Natl Acad Sci U S A. 1989 Feb;86(3):921–924. doi: 10.1073/pnas.86.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann W., Kaiser I., Jakschik B. A. The sensitized liver represents a rich source of endogenous leukotrienes. Hepatology. 1991 Mar;13(3):482–488. [PubMed] [Google Scholar]
- Hagmann W., Parthé S., Kaiser I. Uptake, production and metabolism of cysteinyl leukotrienes in the isolated perfused rat liver. Inhibition of leukotriene uptake by cyclosporine. Biochem J. 1989 Jul 15;261(2):611–616. doi: 10.1042/bj2610611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Wood K. W., Mamon H. J., Okuda K., Roberts T. M., Griffin J. D. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce rapid phosphorylation and activation of the proto-oncogene Raf-1 in a human factor-dependent myeloid cell line. Blood. 1991 Jan 15;77(2):243–248. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kargman S., Rouzer C. A. Studies on the regulation, biosynthesis, and activation of 5-lipoxygenase in differentiated HL60 cells. J Biol Chem. 1989 Aug 5;264(22):13313–13320. [PubMed] [Google Scholar]
- Kargman S., Vickers P. J., Evans J. F. A23187-induced translocation of 5-lipoxygenase in osteosarcoma cells. J Cell Biol. 1992 Dec;119(6):1701–1709. doi: 10.1083/jcb.119.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto Y., De Weck A. L., Dahinden C. A. The effect of interleukin 3 upon IgE-dependent and IgE-independent basophil degranulation and leukotriene generation. Eur J Immunol. 1991 Feb;21(2):361–368. doi: 10.1002/eji.1830210217. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern M. R., Mong S., Mong S. M., Bartus J. O., Sarau H. M., Clark M. A., Foley J. J., Crooke S. T. Transient activation of topoisomerase I in leukotriene D4 signal transduction in human cells. Biochem J. 1990 Jan 1;265(1):101–107. doi: 10.1042/bj2650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Léveillé C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990 Jan 18;343(6255):278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Bryant R. W., Coscolluela C., Myers R. F., Siegel M. I. Ionophore-stimulated lipoxygenase activity and histamine release in a cloned murine mast cell, MC9. Prostaglandins. 1985 Mar;29(3):405–430. doi: 10.1016/0090-6980(85)90099-1. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Klemba M. W., Munday N. A., Zamboni R. J., Ford-Hutchinson A. W. Human leukotriene C4 synthase expression in dimethyl sulfoxide-differentiated U937 cells. J Biol Chem. 1992 Sep 5;267(25):17849–17857. [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Odlander B., Jakobsson P. J., Medina J. F., Rådmark O., Yamaoka K. A., Rosén A., Claesson H. E. Formation and effects of leukotriene B4 in human lymphocytes. Int J Tissue React. 1989;11(6):277–289. [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., den Hertog J., de Laat S. W. Epidermal growth factor activates calcium channels by phospholipase A2/5-lipoxygenase-mediated leukotriene C4 production. Cell. 1992 Apr 17;69(2):295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Austen K. F., Caulfield J. P., Hein A., Liu F. T., Clabby M., Nabel G., Cantor H., Friedman S. Cloned mouse mast cells derived from immunized lymph node cells and from foetal liver cells exhibit characteristics of bone marrow-derived mast cells containing chondroitin sulphate E proteoglycan. Immunology. 1984 Jul;52(3):563–575. [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem. 1988 Aug 5;263(22):10980–10988. [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schonhardt T., Ferber E. Translocation of phospholipase A2 from cytosol to membranes induced by 1-oleoyl-2-acetyl-glycerol in serum-free cultured macrophages. Biochem Biophys Res Commun. 1987 Dec 16;149(2):769–775. doi: 10.1016/0006-291x(87)90434-7. [DOI] [PubMed] [Google Scholar]
- Stanková J., Rola-Pleszczynski M. Leukotriene B4 stimulates c-fos and c-jun gene transcription and AP-1 binding activity in human monocytes. Biochem J. 1992 Mar 15;282(Pt 3):625–629. doi: 10.1042/bj2820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Mizuno S., Kawai S. Inhibition of transforming activity of tyrosine kinase oncogenes by herbimycin A. Virology. 1988 May;164(1):294–298. doi: 10.1016/0042-6822(88)90649-6. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Lyall R., Jariwala N., Zilberstein A., Haimovich J. Antigen- and ionophore-induced signal transduction in rat basophilic leukemia cells involves protein tyrosine phosphorylation. J Biol Chem. 1991 Nov 25;266(33):22564–22568. [PubMed] [Google Scholar]


