Abstract
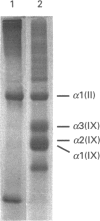
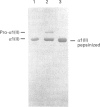
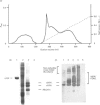
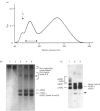
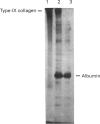
We report for the first time that, after centrifugation of adult bovine vitreous, the hyaluronan-rich supernatant contains collagens which can be isolated in their intact forms by precipitation with 4.5 M NaCl. This precipitate constituted approx. 4% of the total vitreous collagen and comprised collagen types IX and II (in the approximate ratio of 4:1) with negligible amounts of type-V/XI collagen. Type-II collagen was present partly in a pro-alpha 1(II) form, suggesting that there is active synthesis of type-II collagen into the matrix of adult bovine vitreous. Type-IX collagen was purified (2-2.5 mg/l of vitreous) and its glycosaminoglycan chain composition was analysed. Bovine vitreous type-IX collagen always possessed a glycosaminoglycan chain of comparatively low M(r) that was predominantly 4-sulphated, with chondroitin 6-sulphate representing a more minor component. By contrast, chick vitreous has been shown to contain type-IX collagen which always possesses a high-M(r) chondroitin sulphate chain that is predominantly 6-sulphated. The functional significance of these different glycosaminoglycan chain lengths and sulphation patterns is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. S., Otterbein E. C., Wardi A. H. Isolation and characterization of the sulfated glycosaminoglycans of the vitreous body. Biochim Biophys Acta. 1977 Jun 23;498(1):167–175. doi: 10.1016/0304-4165(77)90097-6. [DOI] [PubMed] [Google Scholar]
- Ayad S., Marriott A., Brierley V. H., Grant M. E. Mammalian cartilage synthesizes both proteoglycan and non-proteoglycan forms of type IX collagen. Biochem J. 1991 Sep 1;278(Pt 2):441–445. doi: 10.1042/bj2780441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S., Marriott A., Morgan K., Grant M. E. Bovine cartilage types VI and IX collagens. Characterization of their forms in vivo. Biochem J. 1989 Sep 15;262(3):753–761. doi: 10.1042/bj2620753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S., Weiss J. B. A new look at vitreous-humour collagen. Biochem J. 1984 Mar 15;218(3):835–840. doi: 10.1042/bj2180835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Fitch J. M., Babiarz J. P., Linsenmayer T. F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988 Mar;106(3):999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
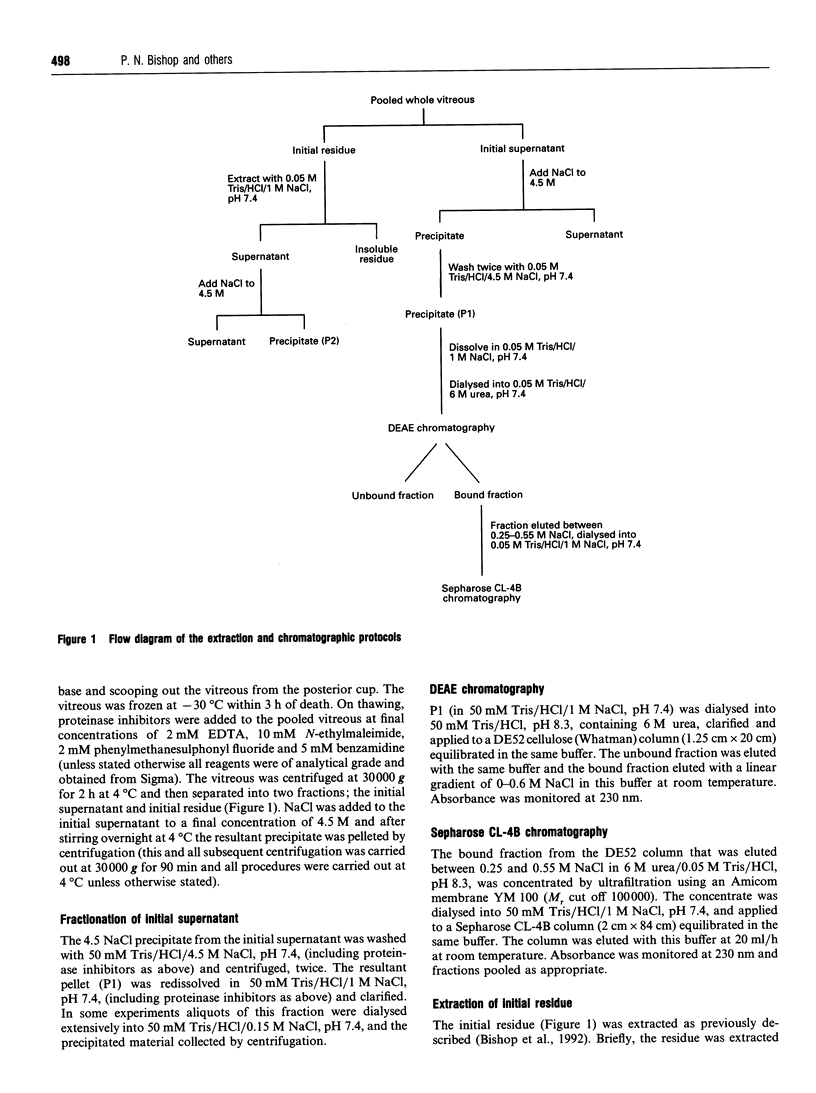
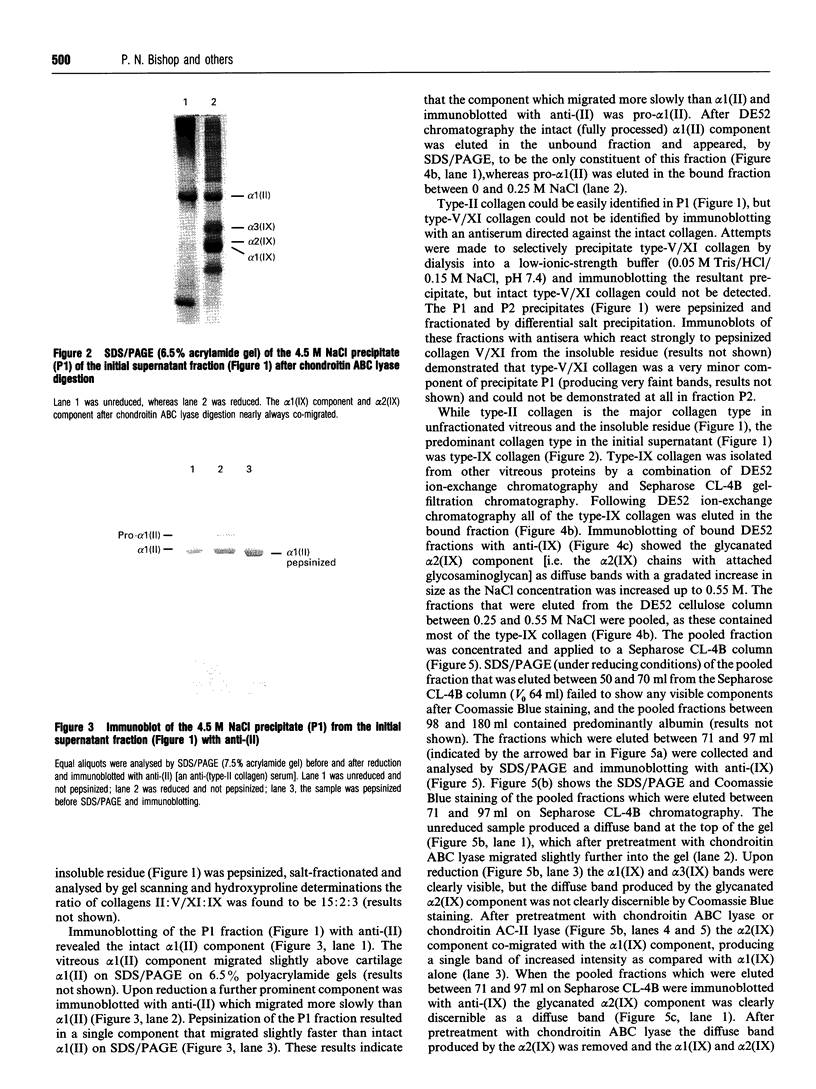
- Bishop P., McLeod D., Ayad S. Extraction and characterisation of the intact form of bovine vitreous type IX collagen. Biochem Biophys Res Commun. 1992 May 29;185(1):392–397. doi: 10.1016/s0006-291x(05)80998-2. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brewton R. G., Mayne R. Mammalian vitreous humor contains networks of hyaluronan molecules: electron microscopic analysis using the hyaluronan-binding region (G1) of aggrecan and link protein. Exp Cell Res. 1992 Feb;198(2):237–249. doi: 10.1016/0014-4827(92)90376-j. [DOI] [PubMed] [Google Scholar]
- Brewton R. G., Ouspenskaia M. V., van der Rest M., Mayne R. Cloning of the chicken alpha 3(IX) collagen chain completes the primary structure of type IX collagen. Eur J Biochem. 1992 Apr 15;205(2):443–449. doi: 10.1111/j.1432-1033.1992.tb16798.x. [DOI] [PubMed] [Google Scholar]
- Brewton R. G., Wright D. W., Mayne R. Structural and functional comparison of type IX collagen-proteoglycan from chicken cartilage and vitreous humor. J Biol Chem. 1991 Mar 15;266(8):4752–4757. [PubMed] [Google Scholar]
- Bruckner P., Vaughan L., Winterhalter K. H. Type IX collagen from sternal cartilage of chicken embryo contains covalently bound glycosaminoglycans. Proc Natl Acad Sci U S A. 1985 May;82(9):2608–2612. doi: 10.1073/pnas.82.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. L., Osborne D. J. The separation of chondroitin sulfate disaccharides and hyaluronan oligosaccharides by capillary zone electrophoresis. Anal Biochem. 1991 May 15;195(1):132–140. doi: 10.1016/0003-2697(91)90308-g. [DOI] [PubMed] [Google Scholar]
- Har-el R., Sharma Y. D., Aguilera A., Ueyama N., Wu J. J., Eyre D. R., Juricić L., Chandrasekaran S., Li M., Nah H. D. Cloning and developmental expression of the alpha 3 chain of chicken type IX collagen. J Biol Chem. 1992 May 15;267(14):10070–10076. [PubMed] [Google Scholar]
- Irwin M. H., Mayne R. Use of monoclonal antibodies to locate the chondroitin sulfate chain(s) in type IX collagen. J Biol Chem. 1986 Dec 15;261(35):16281–16283. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayne R., Brewton R. G., Mayne P. M., Baker J. R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993 May 5;268(13):9381–9386. [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muragaki Y., Kimura T., Ninomiya Y., Olsen B. R. The complete primary structure of two distinct forms of human alpha 1 (IX) collagen chains. Eur J Biochem. 1990 Sep 24;192(3):703–708. doi: 10.1111/j.1432-1033.1990.tb19279.x. [DOI] [PubMed] [Google Scholar]
- Pan T. C., Zhang R. Z., Mattei M. G., Timpl R., Chu M. L. Cloning and chromosomal location of human alpha 1(XVI) collagen. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6565–6569. doi: 10.1073/pnas.89.14.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle G. A., Dodd C. M., Osborn J. W., Pearson C. H., Mosmann T. R. Production and characterization of monoclonal antibodies to bovine skin proteodermatan sulfate. Coll Relat Res. 1985 Jan;5(1):23–39. doi: 10.1016/s0174-173x(85)80045-5. [DOI] [PubMed] [Google Scholar]
- Ren Z. X., Brewton R. G., Mayne R. An analysis by rotary shadowing of the structure of the mammalian vitreous humor and zonular apparatus. J Struct Biol. 1991 Feb;106(1):57–63. doi: 10.1016/1047-8477(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Sampaio L. de O., Bayliss M. T., Hardingham T. E., Muir H. Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem J. 1988 Sep 15;254(3):757–764. doi: 10.1042/bj2540757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Chen Y., Brass A. Secondary and tertiary structures involving chondroitin and chondroitin sulphates in solution, investigated by rotary shadowing/electron microscopy and computer simulation. Eur J Biochem. 1992 Oct 15;209(2):675–680. doi: 10.1111/j.1432-1033.1992.tb17335.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Cummings C., Brass A., Chen Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J. 1991 Mar 15;274(Pt 3):699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. The chemical morphology of the vitreous. Eye (Lond) 1992;6(Pt 6):553–555. doi: 10.1038/eye.1992.120. [DOI] [PubMed] [Google Scholar]
- Seery C. M., Davison P. F. Collagens of the bovine vitreous. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1540–1550. [PubMed] [Google Scholar]
- Snowden J. M., Eyre D. R., Swann D. A. Vitreous structure. VI. Age-related changes in the thermal stability and crosslinks of vitreous, articular cartilage and tendon collagens. Biochim Biophys Acta. 1982 Sep 7;706(2):153–157. doi: 10.1016/0167-4838(82)90481-2. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Constable I. J. Vitreous structure. I. Distribution of hyaluronate and protein. Invest Ophthalmol. 1972 Mar;11(3):159–163. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley E. A., Roth S. Interactions between the carbohydrate chains of hyaluronate and chondroitin sulphate. Nature. 1980 Jan 17;283(5744):268–271. doi: 10.1038/283268a0. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988 Mar;106(3):991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Watt S. L., Lunstrum G. P., McDonough A. M., Keene D. R., Burgeson R. E., Morris N. P. Characterization of collagen types XII and XIV from fetal bovine cartilage. J Biol Chem. 1992 Oct 5;267(28):20093–20099. [PubMed] [Google Scholar]
- Wu J. J., Woods P. E., Eyre D. R. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem. 1992 Nov 15;267(32):23007–23014. [PubMed] [Google Scholar]
- Yada T., Arai M., Suzuki S., Kimata K. Occurrence of collagen and proteoglycan forms of type IX collagen in chick embryo cartilage. Production and characterization of a collagen form-specific antibody. J Biol Chem. 1992 May 5;267(13):9391–9397. [PubMed] [Google Scholar]
- Yada T., Suzuki S., Kobayashi K., Kobayashi M., Hoshino T., Horie K., Kimata K. Occurrence in chick embryo vitreous humor of a type IX collagen proteoglycan with an extraordinarily large chondroitin sulfate chain and short alpha 1 polypeptide. J Biol Chem. 1990 Apr 25;265(12):6992–6999. [PubMed] [Google Scholar]
- van der Rest M., Dublet B., Champliaud M. F. Fibril-associated collagens. Biomaterials. 1990 Jul;11:28–31. [PubMed] [Google Scholar]