Abstract
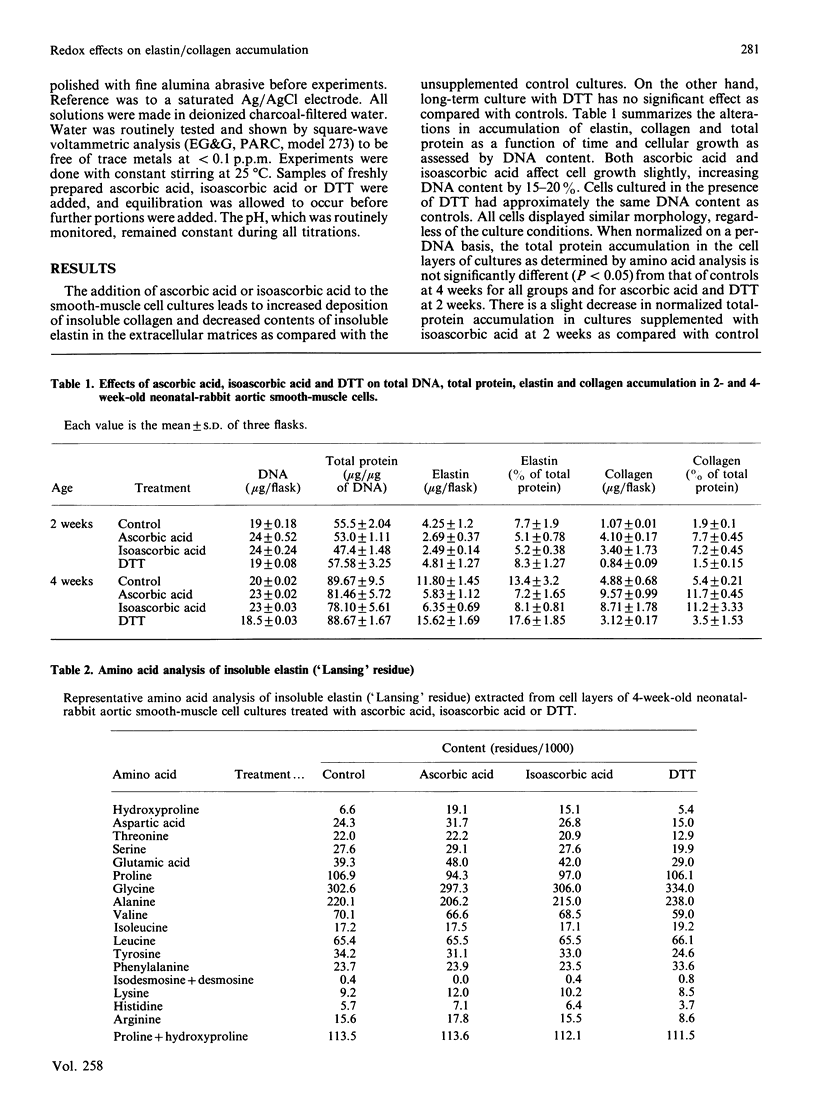
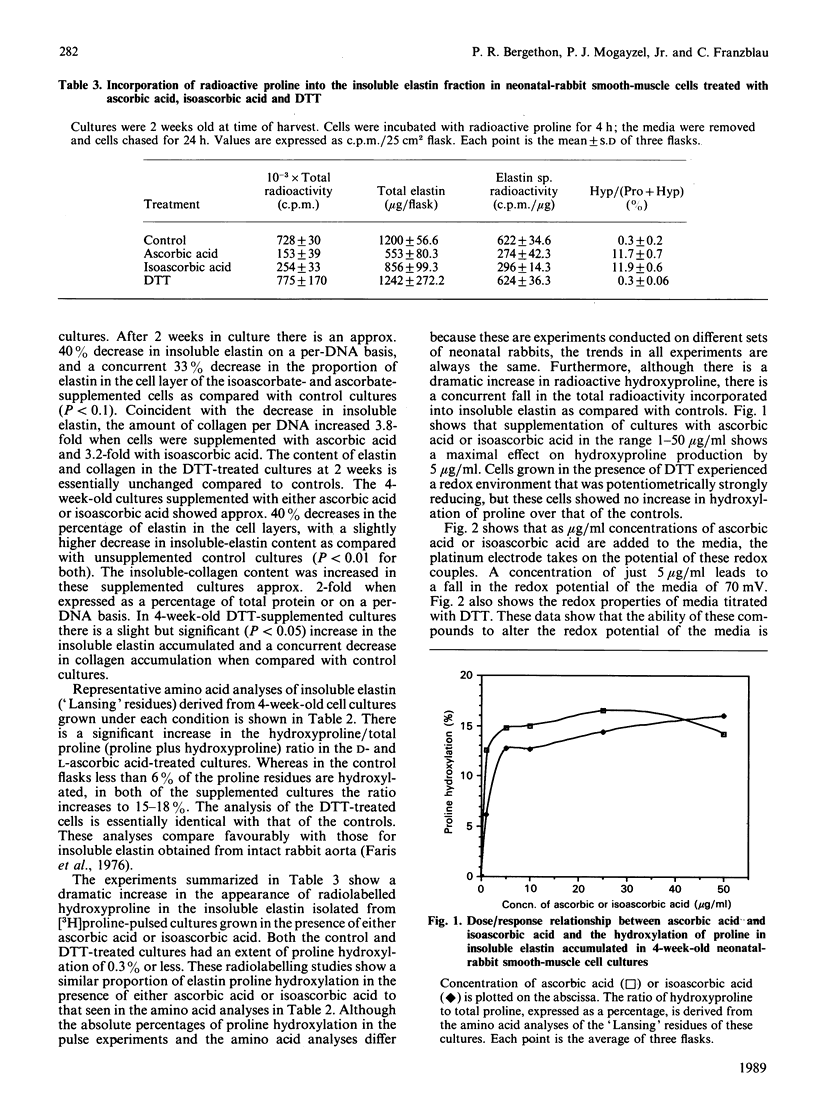
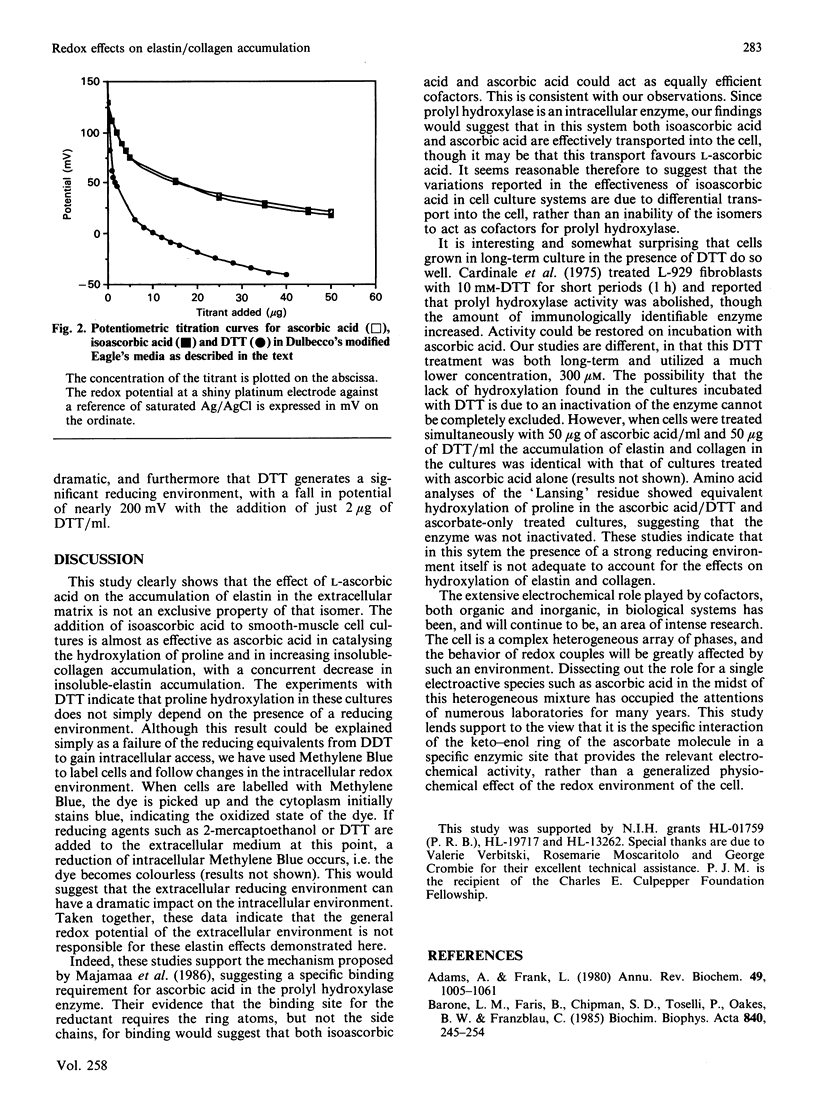
We show here that cultured neonatal-rabbit aortic smooth-muscle cells produce and accumulate significant amounts of insoluble elastin. When grown in the presence of ascorbic acid, the amount of insoluble elastin in these cultures decreases, whereas the accumulation of collagen increases. These changes have been attributed to increased hydroxylation of proline in elastin. The function of ascorbic acid in proline hydroxylation is thought to be that of a reductive cofactor that maintains the proper oxidation state of molecular iron in the enzyme complex. This study shows that both ascorbic and isoascorbic acids act similarly to modify the accumulation of elastin and collagen in culture. On the other hand, cultures grown in the presence of dithiothreitol, a reducing agent previously shown to act as a cofactor for prolyl hydroxylase, do not demonstrate altered elastin accumulation. These studies are consistent with the suggestion that there is a specific role for ascorbic acid in this cellular system that cannot be replaced by other reducing cofactors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone L. M., Faris B., Chipman S. D., Toselli P., Oakes B. W., Franzblau C. Alteration of the extracellular matrix of smooth muscle cells by ascorbate treatment. Biochim Biophys Acta. 1985 Jun 18;840(2):245–254. doi: 10.1016/0304-4165(85)90125-4. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Cardinale G. J., Stassen F. L., Kuttan R., Udenfriend S. Activation of prolyl hydroxylase in fibroblasts by ascorbic acid. Ann N Y Acad Sci. 1975 Sep 30;258:278–287. doi: 10.1111/j.1749-6632.1975.tb29288.x. [DOI] [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Chauhan U., Assad R., Peterkofsky B. Cysteinyl-cysteine and the microsomal protein from which it is derived act as reducing cofactor for prolyl hydroxylase. Biochem Biophys Res Commun. 1985 Aug 30;131(1):277–283. doi: 10.1016/0006-291x(85)91799-1. [DOI] [PubMed] [Google Scholar]
- Doner L. W., Hicks K. B. High-performance liquid chromatographic separation of ascorbic acid, erythorbic acid, dehydroascorbic acid, dehydroerythorbic acid, diketogulonic acid, and diketogluconic acid. Anal Biochem. 1981 Jul 15;115(1):225–230. doi: 10.1016/0003-2697(81)90550-9. [DOI] [PubMed] [Google Scholar]
- Dunn D. M., Franzblau C. Effects of ascorbate on insoluble elastin accumulation and cross-link formation in rabbit pulmonary artery smooth muscle cultures. Biochemistry. 1982 Aug 31;21(18):4195–4202. doi: 10.1021/bi00261a001. [DOI] [PubMed] [Google Scholar]
- Englard S., Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- Faris B., Ferrera R., Toselli P., Nambu J., Gonnerman W. A., Franzblau C. Effect of varying amounts of ascorbate on collagen, elastin and lysyl oxidase synthesis in aortic smooth muscle cell cultures. Biochim Biophys Acta. 1984 Jan 24;797(1):71–75. doi: 10.1016/0304-4165(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Faris B., Salcedo L. L., Cook V., Johnson L., Foster J. A., Franzblau C. The synthesis of connective tissue protein in smooth muscle cells. Biochim Biophys Acta. 1976 Jan 5;418(1):93–103. doi: 10.1016/0005-2787(76)90330-0. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Faris B. Biosynthesis of insoluble elastin in cell and organ cultures. Methods Enzymol. 1982;82(Pt A):615–637. doi: 10.1016/0076-6879(82)82091-0. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- LANSING A. I., ROSENTHAL T. B., ALEX M., DEMPSEY E. W. The structure and chemical characterization of elastic fibers as revealed by elastase and by electron microscopy. Anat Rec. 1952 Dec;114(4):555–575. doi: 10.1002/ar.1091140404. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Murad S., Grove D., Lindberg K. A., Reynolds G., Sivarajah A., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981 May;78(5):2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Oakes B. W., Batty A. C., Handley C. J., Sandberg L. B. The synthesis of elastin, collagen, and glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur J Cell Biol. 1982 Apr;27(1):34–46. [PubMed] [Google Scholar]
- PETERKOFSKY B., UDENFRIEND S. ENZYMATIC HYDROXYLATION OF PROLINE IN MICROSOMAL POLYPEPTIDE LEADING TO FORMATION OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Feb;53:335–342. doi: 10.1073/pnas.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest R. E., Bublitz C. The influence of ascorbic acid and tetrahydropteridine on the synthesis of hydroxyproline by cultured cells. Lab Invest. 1967 Oct;17(4):371–379. [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Purification and properties of collagen proline hydroxylase from newborn rat skin. Arch Biochem Biophys. 1970 Aug;139(2):329–339. doi: 10.1016/0003-9861(70)90485-6. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Davies P. J., Gevers W. Elastin biosynthesis by smooth muscle cells cultured under scorbutic conditions. Biochem Biophys Res Commun. 1979 Dec 14;91(3):739–746. doi: 10.1016/0006-291x(79)91942-9. [DOI] [PubMed] [Google Scholar]
- de Clerck Y. A., Jones P. A. The effect of ascorbic acid on the nature and production of collagen and elastin by rat smooth-muscle cells. Biochem J. 1980 Jan 15;186(1):217–225. doi: 10.1042/bj1860217. [DOI] [PMC free article] [PubMed] [Google Scholar]


