Abstract
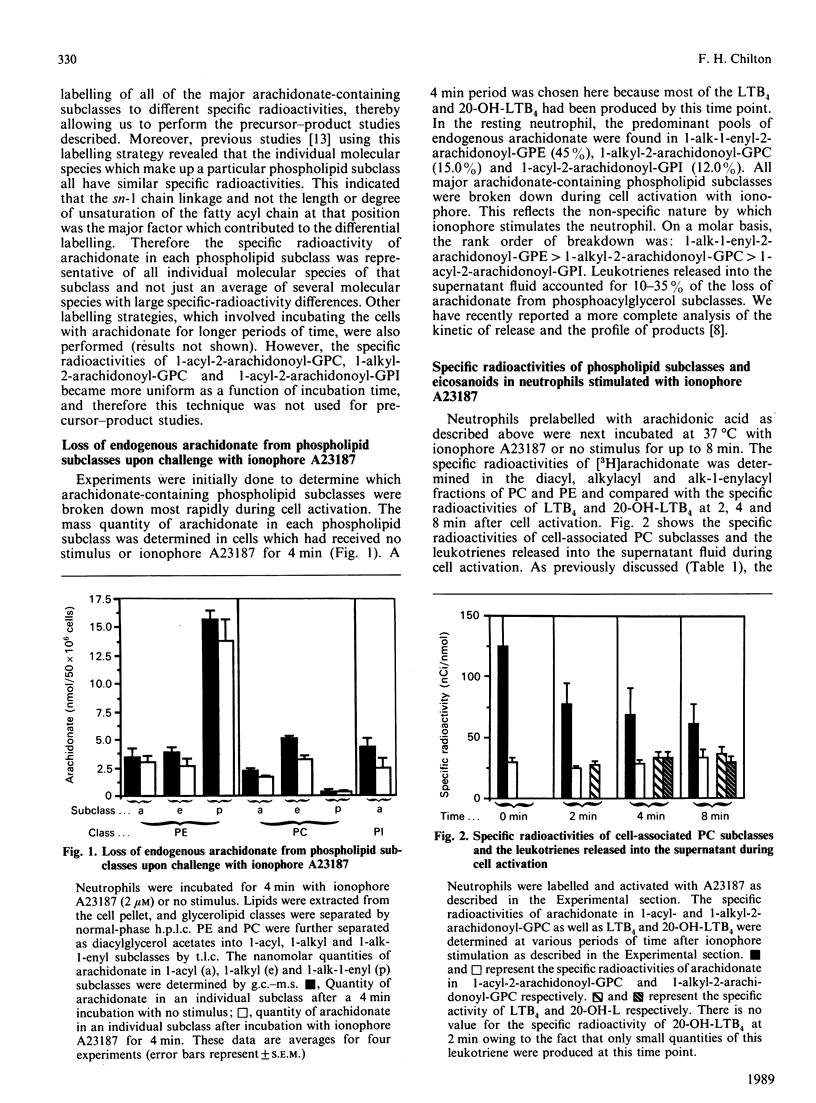
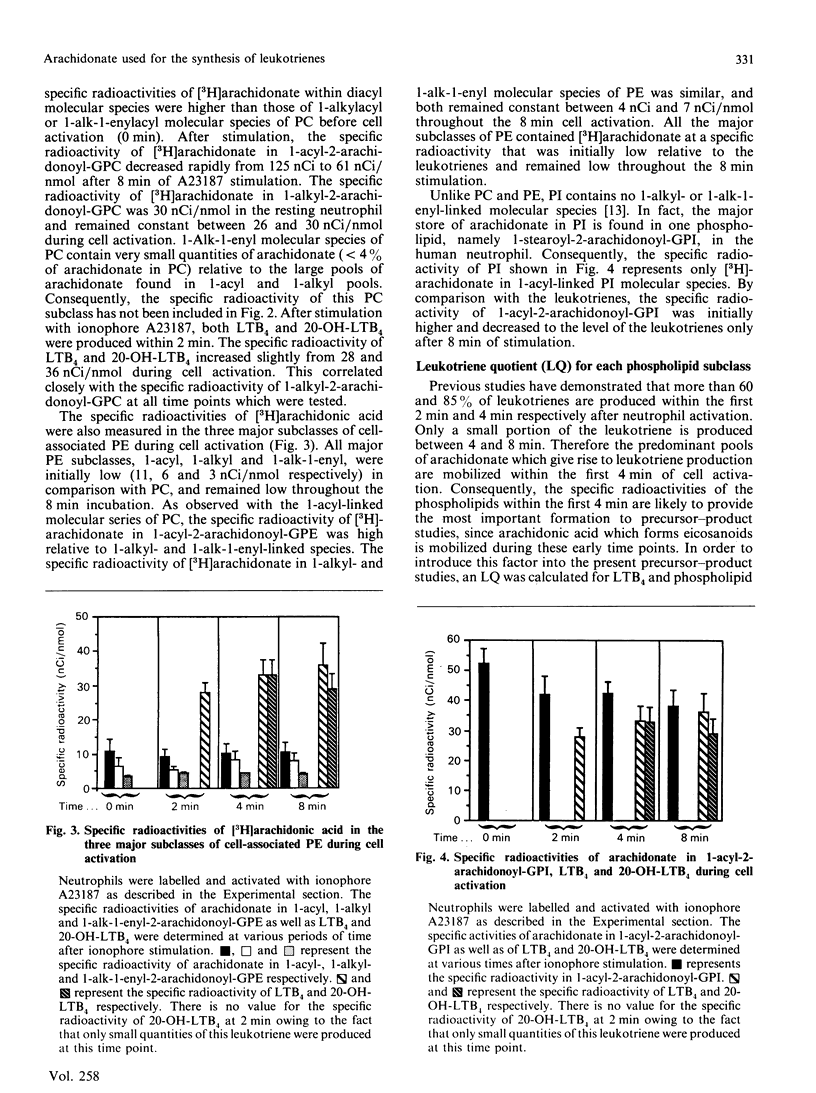
The present study has employed two approaches to address the question of whether there are specific phospholipid sources of arachidonate used for leukotriene biosynthesis in the human neutrophil. Firstly, g.c.-m.s. analysis indicated that arachidonate was lost from all major arachidonate-containing phospholipid subclasses during cell activation with ionophore A23187. On a molar basis, the rank order of breakdown among the three major phospholipids was: 1-alk-1-enyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine greater than 1-alkyl-2-arachidonoyl-sn-3-phosphocholine greater than 1-acyl-2-arachidonyl-sn-3-phosphoinositol. Leukotrienes released into the supernatant fluid accounted for only 10-35% of the total arachidonate depletion. Phospholipid sources were also identified in labelling experiments where the specific radioactivity of arachidonate in phospholipid subclasses, as well as leukotrienes produced during cell activation, was measured. The specific radioactivity of arachidonate within 1-acyl-linked molecular species of phosphatidylcholine and phosphatidylinositol was initially high relative to the leukotrienes and decreased rapidly with stimulation. By contrast, the specific radioactivity of arachidonate in all three subclasses of phosphatidylethanolamine, 1-acyl, 1-alkyl, and 1-alk-1-enyl, was 3-5-fold below that of the leukotrienes throughout cell activation. Of the six major arachidonate-containing subclasses, only in the case of 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine did the specific radioactivity correlate well with that of leukotriene B4 and 20-hydroxyleukotriene B4. These data strongly suggest that 1-ether-linked phospholipids are an important source of arachidonate used for leukotriene biosynthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Snyder F. Release of arachidonic acid from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine, a precursor of platelet-activating factor, in rat alveolar macrophages. Biochim Biophys Acta. 1984 Oct 24;796(1):92–101. doi: 10.1016/0005-2760(84)90242-x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chilton F. H., Connell T. R. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J Biol Chem. 1988 Apr 15;263(11):5260–5265. [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Chilton F. H., Hadley J. S., Murphy R. C. Incorporation of arachidonic acid into 1-acyl-2-lyso-sn-glycero-3-phosphocholine of the human neutrophil. Biochim Biophys Acta. 1987 Jan 13;917(1):48–56. doi: 10.1016/0005-2760(87)90282-7. [DOI] [PubMed] [Google Scholar]
- Chilton F. H., Murphy R. C. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem. 1986 Jun 15;261(17):7771–7777. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Ellis J. M., Swendsen C. L., Wykle R. L. Selective acylation of lyso platelet activating factor by arachidonate in human neutrophils. J Biol Chem. 1983 Jun 25;258(12):7268–7271. [PubMed] [Google Scholar]
- Clay K. L., Murphy R. C., Andres J. L., Lynch J., Henson P. M. Structure elucidation of platelet activating factor derived from human neutrophils. Biochem Biophys Res Commun. 1984 Jun 29;121(3):815–825. doi: 10.1016/0006-291x(84)90751-4. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S. Chemiluminescence of human neutrophils induced by soluble stimuli: effect of divalent cations. Infect Immun. 1982 Jan;35(1):206–212. doi: 10.1128/iai.35.1.206-212.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie C. C., Detty D. M. Arachidonic acid turnover in response to lipopolysaccharide and opsonized zymosan in human monocyte-derived macrophages. Biochem J. 1986 May 15;236(1):251–259. doi: 10.1042/bj2360251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews W. R., Rokach J., Murphy R. C. Analysis of leukotrienes by high-pressure liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):96–101. doi: 10.1016/0003-2697(81)90162-7. [DOI] [PubMed] [Google Scholar]
- Mueller H. W., O'Flaherty J. T., Greene D. G., Samuel M. P., Wykle R. L. 1-O-alkyl-linked glycerophospholipids of human neutrophils: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. J Lipid Res. 1984 Apr;25(4):383–388. [PubMed] [Google Scholar]
- Mueller H. W., Purdon A. D., Smith J. B., Wykle R. L. 1-O-alkyl-linked phosphoglycerides of human platelets: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. Lipids. 1983 Nov;18(11):814–819. doi: 10.1007/BF02534641. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Kurihara K., Sugiura T., Waku K. Relative degradation of different arachidonoyl molecular species of choline glycerophospholipids in opsonized zymosan-stimulated rabbit alveolar macrophages. Biochim Biophys Acta. 1986 May 21;876(3):601–610. [PubMed] [Google Scholar]
- Nakagawa Y., Waku K., Ishima Y. Changes in the composition of fatty chains of diacyl, alkylacyl and alkenylacyl ethanolamine and choline phosphoglycerides during the development of chick heart ventricular cells. High accumulation of 22-carbon fatty acid in ether phospholipids. Biochim Biophys Acta. 1982 Sep 14;712(3):667–676. [PubMed] [Google Scholar]
- Patton G. M., Fasulo J. M., Robins S. J. Separation of phospholipids and individual molecular species of phospholipids by high-performance liquid chromatography. J Lipid Res. 1982 Jan;23(1):190–196. [PubMed] [Google Scholar]
- Sugiura T., Katayama O., Fukui J., Nakagawa Y., Waku K. Mobilization of arachidonic acid between diacyl and ether phospholipids in rabbit alveolar macrophages. FEBS Lett. 1984 Jan 9;165(2):273–276. doi: 10.1016/0014-5793(84)80184-2. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Masuzawa Y., Waku K. Alkenyl and alkyl ether phospholipids in pig mesenteric lymph node lymphocytes. Lipids. 1980 Jun;15(6):475–478. doi: 10.1007/BF02534076. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Soga N., Nitta H., Waku K. Occurrence of alkyl ether phospholipids in rabbit platelets: compositions and fatty chain profiles. J Biochem. 1983 Nov;94(5):1719–1722. [PubMed] [Google Scholar]
- Swendsen C. L., Ellis J. M., Chilton F. H., 3rd, O'Flaherty J. T., Wykle R. L. 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine: a novel source of arachidonic acid in neutrophils stimulated by the calcium ionophore A23187. Biochem Biophys Res Commun. 1983 May 31;113(1):72–79. doi: 10.1016/0006-291x(83)90433-3. [DOI] [PubMed] [Google Scholar]
- Tou J. S. Incorporation of arachidonic acid and eicosapentaenoic acid into phospholipids by polymorphonuclear leukocytes in vitro. Lipids. 1984 Aug;19(8):573–577. doi: 10.1007/BF02534713. [DOI] [PubMed] [Google Scholar]
- Tou J. S. Platelet-activating factor modulates phospholipid acylation in human neutrophils. Lipids. 1987 May;22(5):333–337. doi: 10.1007/BF02534002. [DOI] [PubMed] [Google Scholar]
- Yoshioka S., Nakashima S., Okano Y., Hasegawa H., Ichiyama A., Nozawa Y. Phospholipid (diacyl, alkylacyl, alkenylacyl) and fatty acyl chain composition in murine mastocytoma cells. J Lipid Res. 1985 Sep;26(9):1134–1141. [PubMed] [Google Scholar]