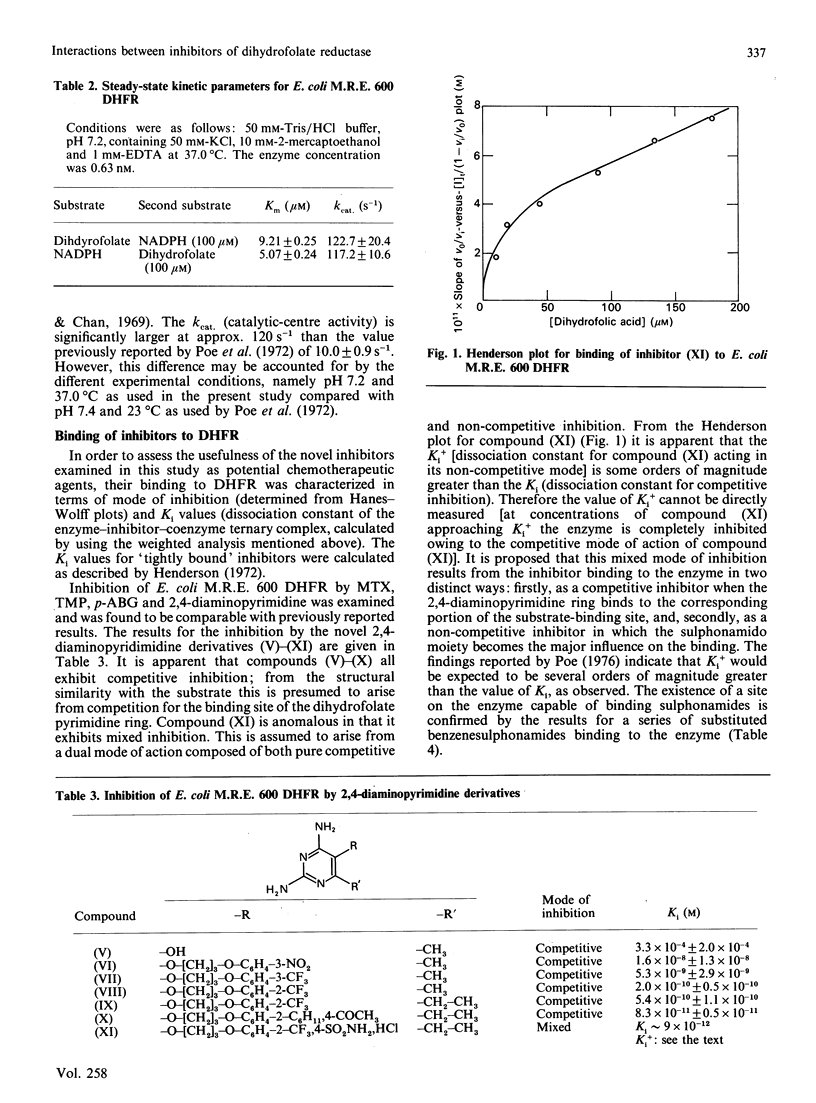
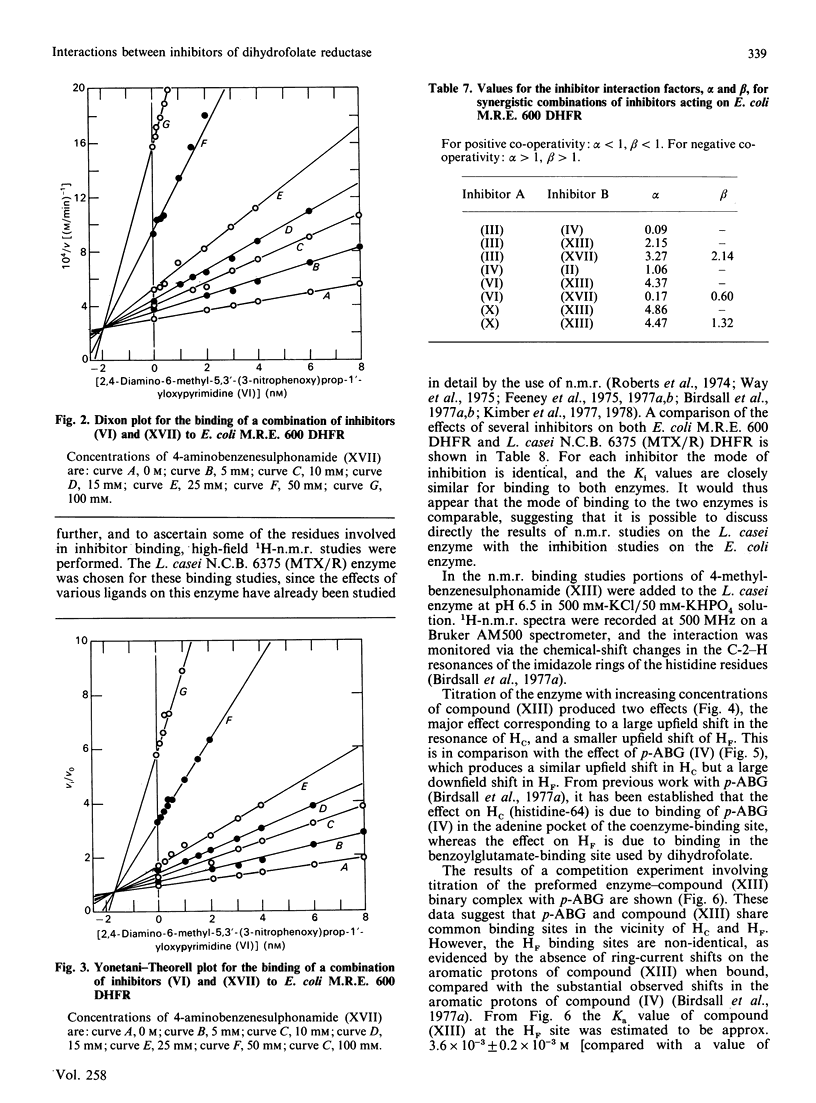
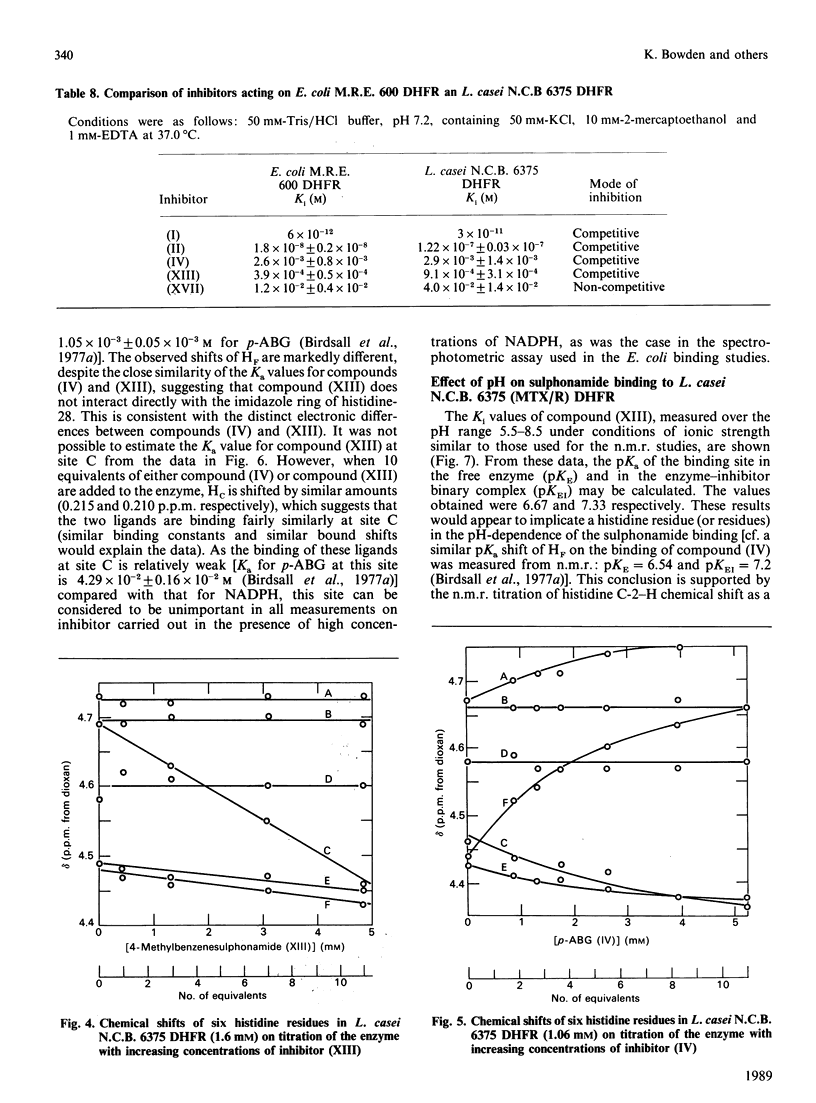
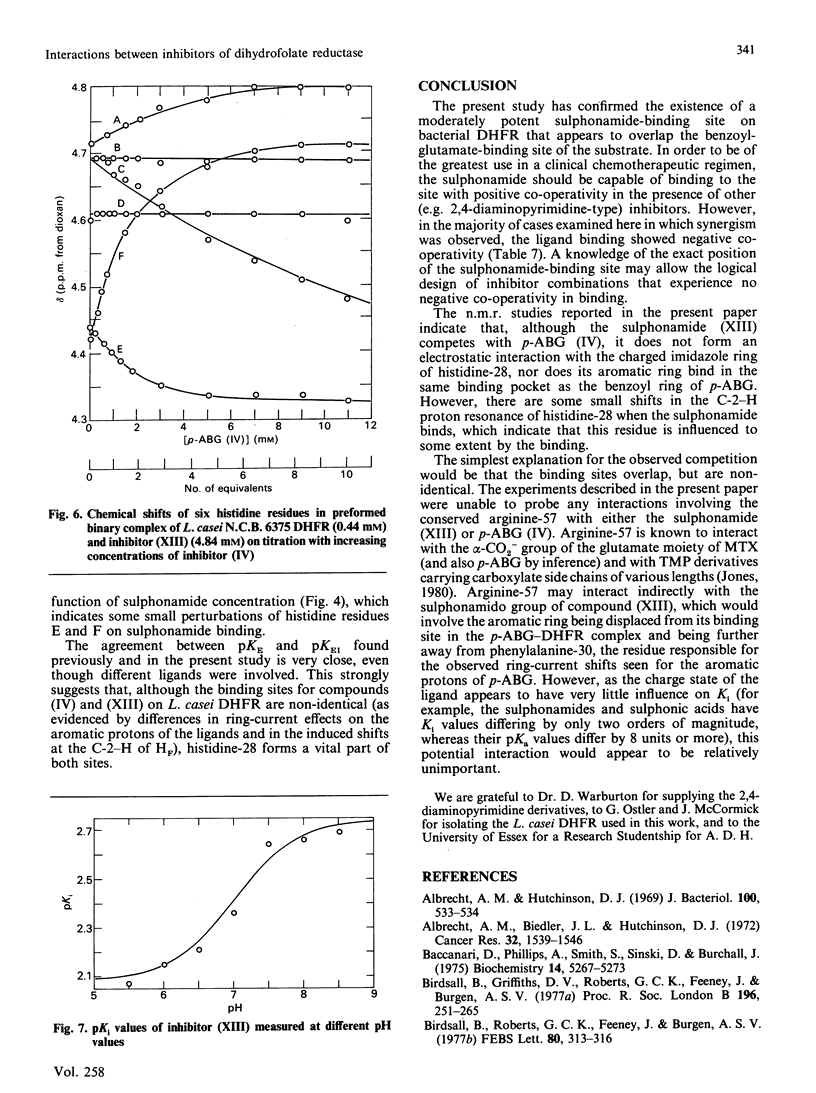
Abstract
The binding of substrates and inhibitors to dihydrofolate reductase was studied by steady-state kinetics and high-field 1H-n.m.r. spectroscopy. A series of 5-substituted 2,4-diaminopyrimidines were examined and were found to be 'tightly binding' inhibitors of the enzyme (Ki less than 10(-9) M). Studies on the binding of 4-substituted benzenesulphonamides and benzenesulphonic acids also established the existence of a 'sulphonamide-binding site' on the enzyme. Subsequent n.m.r. experiments showed that there are two binding sites for the sulphonamides on the enzyme, one of which overlaps the coenzyme (NADPH) adenine-ring-binding site. An examination of the pH-dependence of the binding of sulphonamides to the enzyme indicated the influence of an ionizable group on the enzyme that was not directly involved in the sulphonamide binding. The change in pKa value from 6.7 to 7.2 observed on sulphonamide binding suggests the involvement of a histidine residue, which could be histidine-28.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht A. M., Biedler J. L., Hutchison D. J. Two different species of dihydrofolate reductase in mammalian cells differentially resistant to amethopterin and methasquin. Cancer Res. 1972 Jul;32(7):1539–1546. [PubMed] [Google Scholar]
- Albrecht A. M., Hutchison D. J. Folate reductase and specific dihydrofolate reductase activities of the amethopterin-sensitive Streptococcus faecium var. durans. J Bacteriol. 1969 Oct;100(1):533–534. doi: 10.1128/jb.100.1.533-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccanari D., Phillips A., Smith S., Sinski D., Burchall J. Purification and properties of Escherichia coli dihydrofolate reductase. Biochemistry. 1975 Dec 2;14(24):5267–5273. doi: 10.1021/bi00695a006. [DOI] [PubMed] [Google Scholar]
- Birdsall B., Griffiths D. V., Roberts G. C., Feeney J., Burgen A. 1H nuclear magnetic resonance studies of Lactobacillus casei dihydrofolate reductase: effects of substrate and inhibitor binding on the histidine residues. Proc R Soc Lond B Biol Sci. 1977 Mar 18;196(1124):251–265. doi: 10.1098/rspb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Birdsall B., Roberts G. C., Feeney J., Burgen A. S. 31P NMR studies of the binding of adenosine-2'-phosphate to Lactobacillus casei dihydrofolate reductase. FEBS Lett. 1977 Aug 15;80(2):313–316. doi: 10.1016/0014-5793(77)80465-1. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- D'Souza L., Warwick P. E., Freisheim J. H. Purification and properties of dihydrofolate reductase from an amethopterin-resistant strain of Streptococcus faecium. Biochemistry. 1972 Apr 11;11(8):1528–1534. doi: 10.1021/bi00758a030. [DOI] [PubMed] [Google Scholar]
- Dann J. G., Ostler G., Bjur R. A., King R. W., Scudder P., Turner P. C., Roberts G. C., Burgen A. S. Large-scale purification and characterization of dihydrofolate reductase from a methotrexate-resistant strain of Lactobacillus casei. Biochem J. 1976 Sep 1;157(3):559–571. doi: 10.1042/bj1570559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap R. B., Harding N. G., Huennekens F. M. Thymidylate synthetase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1971 Jan 5;10(1):88–97. doi: 10.1021/bi00777a014. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Mathews C. K. Dihydrofolate reductases of Escherichia coli and bacteriophage Tr. A spectrofluorometric study. Biochemistry. 1973 Jan 30;12(3):372–380. doi: 10.1021/bi00727a002. [DOI] [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- Feeney J., Birdsall B., Roberts G. C., Burgen A. S. 31P NMR studies of NADPH and NADP+ binding to L. casei dihydrofolate reductase. Nature. 1975 Oct 16;257(5527):564–566. doi: 10.1038/257564a0. [DOI] [PubMed] [Google Scholar]
- Feeney J., Roberts G. C., Birdsall B., Griffiths D. V., King R. W., Scudder P., Burgen A. 1H nuclear magnetic resonance studies of the tyrosine residues of selectively deuterated Lactobacillus casei dihydrofolate reductase. Proc R Soc Lond B Biol Sci. 1977 Mar 18;196(1124):267–290. doi: 10.1098/rspb.1977.0041. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- GREENBERG J. The antimalarial activity of 2,4-diamino-6,7-diphenyl pterin; its potentiation by sulfadiazine and inhibition by pteroylglutamic acid. J Pharmacol Exp Ther. 1949 Dec;97(4):484–487. [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillcoat B. L., Nixon P. F., Blakley R. L. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal Biochem. 1967 Nov;21(2):178–189. doi: 10.1016/0003-2697(67)90179-0. [DOI] [PubMed] [Google Scholar]
- Hänggi U. J., Littlefield J. W. Isolation and characterization of the multiple forms of dihydrofolate reductase from methotrexate-resistant hamster cells. J Biol Chem. 1974 Mar 10;249(5):1390–1397. [PubMed] [Google Scholar]
- Jackson R. C., Niethammer D. Acquired methotrexate resistance in lymphoblasts resulting from altered kinetic properties of dihydrofoltate reductase. Eur J Cancer. 1977 Jun;13(6):567–575. doi: 10.1016/0014-2964(77)90118-9. [DOI] [PubMed] [Google Scholar]
- Jones T. R. 5-substituted quinazoline antifolates. Eur J Cancer. 1980 May;16(5):707–711. doi: 10.1016/0014-2964(80)90213-3. [DOI] [PubMed] [Google Scholar]
- Kimber B. J., Feeney J., Roberts G. C., Birdsall B., Griffiths D. V., Burgen A. S., Sykes B. D. Proximity of two tryptophan residues in dihydrofolate reductase determined by 19f NMR. Nature. 1978 Jan 12;271(5641):184–185. doi: 10.1038/271184a0. [DOI] [PubMed] [Google Scholar]
- Kimber B. J., Griffiths D. V., Birdsall B., King R. W., Scudder P., Feeney J., Roberts G. C., Burgen A. S. 19 Fnuclear magnetic resonance studies of ligand binding to 3-fluorotyrosine-and 6-fluorotryptophan-containing dihydrofolate reductase from Lactobacillus casei. Biochemistry. 1977 Jul 26;16(15):3492–3500. doi: 10.1021/bi00634a032. [DOI] [PubMed] [Google Scholar]
- Kuyper L. F., Roth B., Baccanari D. P., Ferone R., Beddell C. R., Champness J. N., Stammers D. K., Dann J. G., Norrington F. E., Baker D. J. Receptor-based design of dihydrofolate reductase inhibitors: comparison of crystallographically determined enzyme binding with enzyme affinity in a series of carboxy-substituted trimethoprim analogues. J Med Chem. 1982 Oct;25(10):1120–1122. doi: 10.1021/jm00352a002. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATHEWS C. K., SUTHERLAND K. E. COMPARATIVE BIOCHEMISTRY OF BACTERIAL AND PHAGE-INDUCED DIHYDROFOLATE REDUCTASES. J Biol Chem. 1965 May;240:2142–2147. [PubMed] [Google Scholar]
- Niethammer D., Jackson R. C. Changes of molecular properties associated with the development of resistance against methotrexate in human lymphoblastoid cells. Eur J Cancer. 1975 Dec;11(12):845–854. doi: 10.1016/0014-2964(75)90083-3. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., SIMONSON H. Sequential blocking of metabolic pathways in vivo. Proc Soc Exp Biol Med. 1951 Jan;76(1):41–46. doi: 10.3181/00379727-76-18383. [DOI] [PubMed] [Google Scholar]
- Poe M. Antibacterial synergism: a proposal for chemotherapeutic potentiation between trimethoprim and sulfamethoxazole. Science. 1976 Oct 29;194(4264):533–535. doi: 10.1126/science.788154. [DOI] [PubMed] [Google Scholar]
- Poe M., Greenfield N. J., Hirshfield J. M., Williams M. N., Hoogsteen K. Dihydrofolate reductase. Purification and characterization of the enzyme from an amethopterin-resistant mutant of Escherichia coli. Biochemistry. 1972 Mar 14;11(6):1023–1030. doi: 10.1021/bi00756a012. [DOI] [PubMed] [Google Scholar]
- ROLLO I. M. The mode of action of sulphonamides, proguanil and pyrimethamine on Plasmodium gallinaceum. Br J Pharmacol Chemother. 1955 Jun;10(2):208–214. doi: 10.1111/j.1476-5381.1955.tb00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN R. J., REYNARD A., HANDSCHUMACHER R. E. AN ANALYSIS OF THE LACK OF DRUG SYNERGISM DURING SEQUENTIAL BLOCKADE OF DE NOVO PYRIMIDINE BIOSYNTHESIS. Cancer Res. 1964 Jul;24:1002–1007. [PubMed] [Google Scholar]
- Roberts G. C., Feeney J., Burgen A. S., Yuferov V., Dann J. G., Bjur R. Nuclear magnetic resonance studies of the binding of substrate analogs and coenzyme to dihydrofolate reductase from Lactobacillus casei. Biochemistry. 1974 Dec 17;13(26):5351–5357. doi: 10.1021/bi00723a015. [DOI] [PubMed] [Google Scholar]
- Way J. L., Birdsall B., Feeney J., Roberts G. C., Burgen A. S. A nuclear magnetic resonance study of nicotinamide adenine dinucleotide phosphate binding to Lactobacillus casei dihydrofolate reductase. Biochemistry. 1975 Jul 29;14(15):3470–3475. doi: 10.1021/bi00686a028. [DOI] [PubMed] [Google Scholar]
- Wise E. M., Jr, Abou-Donia M. M. Sulfonamide resistance mechanism in Escherichia coli: R plasmids can determine sulfonamide-resistant dihydropteroate synthases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2621–2625. doi: 10.1073/pnas.72.7.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T., THEORELL H. STUDIES ON LIVER ALCOHOL HYDROGENASE COMPLEXES. 3. MULTIPLE INHIBITION KINETICS IN THE PRESENCE OF TWO COMPETITIVE INHIBITORS. Arch Biochem Biophys. 1964 Jul 20;106:243–251. doi: 10.1016/0003-9861(64)90184-5. [DOI] [PubMed] [Google Scholar]


