Abstract
Background and Objectives
Rapid developments in Alzheimer disease (AD) biomarker research suggest that predictive testing may become widely available. To ensure equal access to AD predictive testing, it is important to understand factors that affect testing interest. Discrimination may influence attitudes toward AD testing, particularly among racially and ethnically minoritized populations, because of structural racism in health care systems. This study examined whether everyday or lifetime discrimination experiences shape interest in AD predictive testing.
Methods
In the 2010 and 2012 biennial Health and Retirement Study waves, respondents were randomly selected to complete questions on interest in receiving free testing that could determine whether they would develop AD in the future. The exposures were everyday discrimination (6 items) and lifetime discrimination (7 items); both were transformed into a binary variable. Logistic regression models predicting interest in AD testing were controlled for deciles of propensity scores for each discrimination measure. Odds ratios were re-expressed as risk differences (RDs).
Results
Our analytic sample included 1,499 respondents. The mean age was 67 (SD = 10.2) years, 57.4% were women, 65.7% were White, and 80% endorsed interest in AD predictive testing. Most of the participants (54.7%) experienced everyday discrimination in at least one domain; 24.1% experienced major lifetime discrimination in at least one domain. Those interested in predictive testing were younger (66 vs 70 years) and more likely to be Black (20% vs 15%) or Latinx (14% vs 8%) than participants uninterested in testing. The probability of wanting an AD test was not associated with discrimination for Black (RD everyday discrimination = −0.026; 95% CI [−0.081 to 0.029]; RD lifetime discrimination = −0.012; 95% CI [−0.085 to 0.063]) or Latinx (RD everyday discrimination = −0.023, 95% CI [−0.082 to 0.039]; RD lifetime discrimination = −0.011; 95% CI [−0.087 to 0.064]) participants.
Discussion
Despite historical and contemporary experiences of discrimination, Black and Latinx individuals express interest in AD testing. However, Black and Latinx individuals remain underrepresented in AD research, including research on AD testing. Interest in personalized information about dementia risk may be a pathway to enhance their inclusion in research and clinical trials.
Introduction
Marked racial disparities exist in Alzheimer disease (AD) misdiagnosis and underdiagnosis.1,2 Among racially and ethnically minoritized communities, diagnosis is often significantly delayed, resulting in more advanced stages of disease and severe cognitive impairment at the time of diagnosis.3,4 Early detection of Alzheimer disease and related dementias (ADRDs) is of growing importance with potential disease-modifying therapies and interventions now emerging.5-7 Biomarker-based predictive testing for AD may soon become widely available. Such testing could enhance early access to care and is likely to become a key pipeline into AD therapeutic research including clinical trials.8-10 Equitable access to early diagnosis is thus vital given the 1.5–2 times greater risk of ADRD among Black and Latinx older adults compared with White individuals.11,12
Both inequitable health care access and differences in interest in diagnostic testing could potentially reduce uptake of AD testing among Black and Latinx adults. Understanding potential drivers of interest in AD diagnostic testing is essential for equitable AD research and clinical practice and requires contextualization of racial group lived experiences within research and the US health care system. For example, the long-standing history of abuse and exploitation of Black individuals within medicine and racism in science understandably creates skepticism and mistrust of the health care system within the Black American community.13-16 The effects of these atrocities remain palpable and affect engagement within the health care system for individuals from racial and ethnically minoritized communities. In addition, Black and Latinx Americans have been egregiously underrepresented in major trials of AD therapeutics and diagnostics, although there have been increased efforts in more recent years (e.g., New Imaging Dementia—Evidence For Amyloid Scanning study). Structural racism and lack of inclusion in prior research may lead to ambivalence about use of AD diagnostic tests among older Black and Latinx adults. Lack of access to health care may also reduce use of diagnostic tests. Recent data show that Black Americans report higher rates of discrimination when seeking health care compared with non-Latinx White individuals and 50% of Black Americans experience barriers to seeking health care.17,18 These experiences may be further compounded by a limited diversity in health care providers, clinician bias in assessment and treatment,19 and a lack of culturally responsive care.20
The relationship between motivations, barriers, and decision-making process for participation in AD biomarker research and predictive testing is complex and remains unclear.21 A critical question is whether encounters with interpersonal discrimination—the unjust and prejudicial treatment of a person based on social strata such as race, age, religion, or other sociodemographic characteristic22—shapes interest in AD diagnostic testing. Discrimination is a well-documented social determinant of health23,24 and may influence attitudes and interest in predictive testing or early diagnosis. In this study, we use national data from middle-aged and older adults to examine whether past experiences of lifetime or everyday discrimination predicts interest in AD testing. We hypothesized that experiences of lifetime and everyday discrimination, above and beyond discrimination experienced directly in a health care setting, reduces interest in AD testing, particularly for individuals from racially and ethnically minoritized communities and those from lower socioeconomic backgrounds. Given the mixed evidence on whether there are sex differences in predictive testing interest,25-27 we also sought to examine whether experiences of sex discrimination affect self-reported interest.
Methods
We included data from the 2010 and 2012 waves of the Health and Retirement Study (HRS). HRS is an ongoing cohort launched in 1992 that surveys around 20,000 adults older than 50 years and their spouses biennially. Details on HRS are documented elsewhere.28 Our sample was restricted to the respondents who were 51 years and older at the time of the 2012 survey and were randomly selected and participated in an experimental module assessing interest in AD testing.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was conducted and approved under University of Michigan Health Sciences/Behavioral Sciences Institutional Review Board (IRB) guidelines (HUM00061128), and all participants provided informed consent. Analyses using the deidentified data were determined exempt by the University of California—San Francisco (UCSF) IRB.
Measures
Primary Outcome
AD Test Willingness
The outcome variable of interest was the respondent's willingness to participate in an AD test, operationalized as a dichotomous variable. In HRS, respondents were asked, “If you could receive a test from your doctor, free of charge, that would definitely determine whether or not you would develop Alzheimer's disease [sometime within the next 5 years/sometime in the future], would you want to be tested?” Participants were randomly assigned to a time frame (next 5 years vs sometime in the future). We conducted a sensitivity analysis and found no difference in the probability of wanting the test between those who answered the question specified as "next 5 years" vs those who answered with "sometime in the future", so responses to these 2 questions were combined. Participant responses were coded as “yes” = 1 and “no” = 0.
Exposures
HRS respondents were randomly assigned to receive questions about everyday discrimination or major lifetime discrimination in 2010 or 2012.
Everyday Discrimination
Interpersonal discrimination in daily life was assessed using the Williams Everyday Discrimination Scale29 and assessed the frequency of everyday discrimination as well as participant-reported attributions for discrimination. The scale included the prompt: “In your day-to-day life, how often have any of the following things happened to you?” and consisted of the following 6 items: (1) You are treated with less courtesy or respect than other people, (2) You receive poorer service than people at restaurants or stores, (3) People act as if you are not smart, (4) People act as if they are afraid of you, (5) You are threatened or harassed, and (6) You receive poorer service or treatment than other people from doctors or hospitals. Possible response options were “never” = 6, “less than once a year” = 5, “a few times a year” = 4, “a few times a month” = 3, “at least once a week” = 2, and “almost every day” = 1.
For data analyses, we created a binary variable such that 1 = respondent said “yes” to at least one of the 7 questions (i.e., ever experienced everyday discrimination) and 0 = respondent said “no” to all the questions (i.e., never experienced everyday discrimination). The frequency of everyday discrimination scale had a Cronbach's alpha of 0.80 for the 2010 HRS wave and 0.83 for the 2012 HRS wave,30 indicating high reliability of this measure among this study sample.
For each of the 6 questions, participants attributed experiences of everyday discrimination to any combination of the following reasons: ancestry or national origin, sex, race, age, religion, weight, physical disability, aspect of physical appearance, sexual orientation, financial status, or other. While race and ancestry are distinct constructs, for this analysis, we considered responses that included either race or ancestry as indicative of experiences of racism. We also considered financial status and sex as a selected reason.
Lifetime Discrimination
Participants were administered a seven-item scale on systemic discrimination across the lifetime.22 The instructions were “For each of the following events, please indicate whether the event occurred at any point in your life.” Lifetime discrimination was the summed affirmatives to the following: (1) You were unfairly dismissed from a job; (2) Not been hired for a job; (3) Unfairly denied a promotion; (4) Unfairly prevented from moving into a neighborhood because the landlord or a realtor refused to sell or rent you a house or apartment; (5) Unfairly denied a bank loan; (6) Unfairly stopped, searched, question, physically threated, or abused by the police; and (7) Unfairly denied health care or treatment. Possible scores ranged from 0 (no lifetime discrimination) to 7 (experienced lifetime discrimination across all 7 domains). For data analyses, we created a binary variable such that 1 = respondent said “yes” to at least one of the 7 questions (i.e., ever experienced lifetime discrimination) and 0 = respondent said “no” to all the questions (i.e., never experienced lifetime discrimination). The Cronbach's alpha for the lifetime discrimination subscale in this study sample was 0.71.
Moderator
Race and ethnicity was evaluated as a moderator. Self-reported race was used in HRS. Participants were asked, What race do you consider yourself to be: White, Black or African American, American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, or something else? Participants were able to provide as many racial categories as they identified with. For those who indicated multiple categories, they were asked to identify the primary racial group they identify with. This response was then recoded into “Black/African American,” “White,”or “Other.” A separate question asked participants, Do you consider yourself Hispanic or Latino? Participants who reported their ethnicity as Hispanic or Latino were included in the “Hispanic/Latinx/Mexican American/Chicanx” group.
From this, 4 racial and ethnic groups were constructed for analyses: Hispanic/Latinx/Mexican American/Chicanx, non-Hispanic Black/African American, non-Hispanic White, and non-Hispanic other race/ethnicity. Non-Hispanic White was the reference group. Given the small size and heterogeneous composition of the ‘other’ racial and ethnic group, results for this group are suppressed but they were included in all models to improve precision of covariate estimates.
Covariates
We controlled for potential confounders: age in years, sex (female vs male), education (high school, <high school), income (), marital status (married/partnered, separated/divorced, widowed/out of house, never married), number of living children, home ownership (yes/no), retired (yes/no), self-report of Medicaid enrollment, urbanicity of residence (urban, suburban, rural), US region of residence (Northeast, Midwest, South, West), born in the United States (vs. not). Sex was completed by self-report. Participants selected from female or male. There were no questions specific to gender identity.
Analytic Strategy
We estimated 6 logistic regressions to evaluate the association between each measure of experiences of discrimination (everyday, lifetime) and interest in receiving an AD test across each racial and ethnic group (Black, Latinx, White). We estimated 3 logistic regressions to evaluate the association between attribution for everyday discrimination (race/ancestry, financial reasons, sex) and interest in receiving an AD test. To account for potential confounders given the relatively small sample size, we estimated propensity scores using logistic regression to predict each exposure (everyday discrimination or lifetime discrimination). Outcome models were then controlled for deciles of propensity scores (estimated using demographic and socioeconomic variables).
In sensitivity analyses, we also applied inverse probability of treatment weights, but the results were substantively similar in all cases, thus these are not included. HRS is a national sample, but we did not apply survey weights to make the sample nationally representative in this analysis. Because odds ratios are often inappropriate when outcomes are very common, we converted odds ratios to risk differences (RDs), defined as the difference in probability of desiring a test for people who had vs had not experienced discrimination.
Given the study's focus on interest in medical predictive testing, we also completed a sensitivity analysis to examine whether there was an association between health care discrimination (i.e., everyday discrimination item: You receive poorer service or treatment than other people from doctors or hospitals; lifetime discrimination item: Unfairly denied health care or treatment) and interest in obtaining an AD predictive test. These 2 questions were combined because only 33 respondents endorsed the lifetime discrimination item on health care.
Data Availability
These data are made publicly available through the Health and Retirement Study (hrs.isr.umich.edu/).
Results
Figure 1 shows our data analytic flowchart resulting in our final sample size of 1,499 participants. Of the participants in the analysis, 1,203 (80.0%) indicated interest in receiving an AD prediction test (Table). More than half of the participants (54.7%) indicated experiencing everyday discrimination in at least one domain, and 24.1% reported experiencing major lifetime discrimination in at least one domain. Participants interested in receiving a test averaged younger age (66 vs 70 years) and were more likely to be Black (20% vs 15%) or Latinx (14% vs 8%) than participants who did not wish to receive a test.
Figure 1. Data Analytic Flowchart Resulting in the Final Sample Size of 1,499 Participants.

Table.
Sample Characteristics by Interest in Receiving Future Alzheimer Disease Testing
|
Sample characteristics (mean, SD) |
Interest in future AD testing | ||
| No (n = 296) | Yes (n = 1,203) | Total (n = 1,499) | |
| Age | 69.7 (10.7) | 65.8 (9.88) | 66.6 (10.2) |
| Sex (#, % female) | 175 (59.1) | 685 (56.9) | 860 (57.4) |
| Sex (#, % male) | 121 (40.9) | 518 (43.1) | 639 (42.6) |
| Race and ethnicity (#, %) | |||
| Hispanic/Latinx/Mexican American/Chicanx | 23 (7.8) | 163 (13.5) | 186 (12.4) |
| Non-Latinx, Black/African American | 44 (14.9) | 234 (19.5) | 278 (18.5) |
| Non-Latinx, White | 222 (75.0) | 763 (63.4) | 985 (65.7) |
| Non-Latinx, other | 7 (2.4) | 43 (3.6) | 50 (3.3) |
| Education (#, % high school graduate or higher) | 226 (76.4) | 932 (77.4) | 1,158 (77.3) |
| Marital status (#, %) | |||
| Married/partnered | 184 (62.2) | 765 (63.6) | 949 (63.3) |
| Separated/divorced | 30 (10.1) | 175 (14.5) | 205 (13.7) |
| Widowed | 67 (22.6) | 206 (17.1) | 273 (18.2) |
| Never married | 15 (5.1) | 57 (4.7) | 72 (4.8) |
| Income (corrected for household size) | 46,558 (69,866) | 42,959 (51,539) | 43,670 (55,496) |
| Everyday discrimination in at least one domain (n, % yes) | 115 (48.9) | 532 (56.2) | 647 (54.7) |
| Lifetime discrimination in at least one domain (n, % yes) | 43 (18.5) | 239 (25.5) | 282 (24.1) |
NOTE: Income was calculated as .
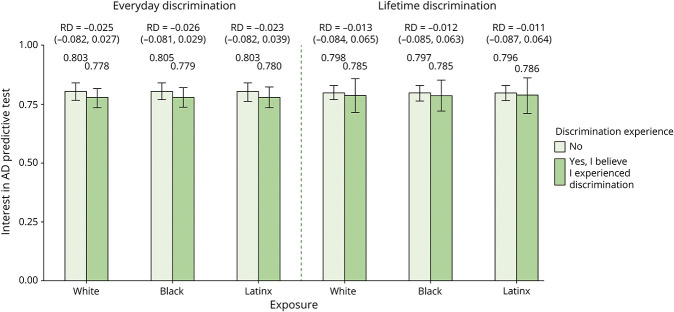
Across all participants who reported experiencing discrimination at least once, the probability of wanting an AD test was not associated with everyday (RD = −0.027, p = 0.362) or lifetime (RD = −0.012, p = 0.77) discrimination. Figure 2 provides an overview of results by racial group. In models adjusted for covariates, the probability of wanting an AD test was not associated with everyday discrimination experiences for respondents who were Black (RD = −0.026; 95% CI [−0.081 to 0.029]) or Latinx (RD = −0.023, 95% CI [−0.082 to 0.039]). A similar pattern was observed for lifetime discrimination such that experiences of discrimination was not associated with likelihood of wanting to receive a test for study participants who were Black (RD = −0.012; 95% CI [−0.085 to 0.063]) or Latinx (RD lifetime discrimination = −0.011; 95% CI [−0.087 to 0.064]).
Figure 2. Probability of Wanting an AD Predictive Test by Experiences of Everyday or Lifetime Discrimination in at Least One Domain Among Black, Latinx, and White Health and Retirement Study Participants.
We next looked at respondents' attributions for everyday discrimination among those who reported experiencing discrimination (Figure 3) and found no evidence that everyday discrimination based on race, ethnicity, or ancestral background (RD = −0.103, 95% CI [−0.295 to 0.076]); financial status (RD = 0.014, 95% CI [−0.081 to 0.097]); or sex (RD = −0.050, 95% CI [−0.223 to 0.074]) was associated with interest in receiving an AD predictive test.
Figure 3. Probability of Wanting an AD Predictive Test by Reason for Experiencing Everyday Discrimination (Race/Ancestry, Financial, and Sex).

Finally, we examined experiences of discrimination within the health care setting specifically and its association with interest in AD predictive testing. Consistent with other findings, the probability of wanting an AD test was not associated with health care discrimination for Black (RD = 0.042, 95% CI [−0.038 to 0.114]) or Latinx (RD = 0.041, 95% CI [−0.043 to 0.114]) respondents.
Discussion
In a national sample of middle-aged and older Americans, most respondents indicated interest in receiving a test for AD. Interest in receiving a test for AD was not associated with past experiences of everyday or major lifetime discrimination, nor was it associated with past experiences of health care discrimination. Specific experiences of everyday discrimination based on racial and ethnic identity or ancestry were not related to interest in receiving a test, nor were experiences of everyday discrimination based on financial status or sex.
Early detection and prevention of ADRDs has elicited great interest among clinicians, researchers, and policymakers. This enthusiasm is enhanced by optimism that disease-modifying therapies may soon be available.5,6 Emerging biomarker, clinical, and neuroimaging-based approaches may help predict whether a cognitively healthy person will eventually develop AD.31,32 With rapid developments in science, dementia predictive testing may soon become a feasible and widely available option32,33 and may help with future planning, advanced care directives, and early implementation of disease-modifying intervention.7 Early predictive testing will also likely provide a critical recruitment pipeline for AD research studies. Given the increased risk of ADRD among Black and Latinx adults,11,12,18 women,18 and those from disadvantaged backgrounds,34-36 achieving equitable access to early diagnosis is essential for early intervention and reduction of AD. Accordingly, identifying factors that affect attitudes toward predictive testing for AD is vital.
Dementia is one of the most feared diseases,37-39 and potential demand for early diagnosis testing for AD is high.40 In 2012, 75% of the US older adults indicated willingness to take a free and definitive test predictive of Alzheimer disease,41 with US Black and Latinx participants more interested in pursuing an early medical test for AD compared with non-Latinx White participants,40,41 which is consistent with the findings of this study. This is in contrast to evidence that White participants were more likely than Black participants to express willingness to enroll in AD biomarker research.42 Actual inclusion of racially and ethnically minoritized persons in AD research remains low.43-45 This is, in part, attributable to historical and contemporary racism and medical mistrust21 and ineffective recruitment methods.46
Our findings must be considered in light of common limitations to observational cohorts. We only have data on a community-dwelling sample, but not on adults who live in institutions or who are unable to participate in HRS because of disabling conditions such as cognitive decline. Although our current analytic sample is more diverse than previous research in this area, respondents to the willingness to take AD testing questions were more than two-thirds non-Latinx, White. The EDS was originally developed for Black American respondents. HRS and many other studies have adapted the EDS instrument for diverse populations, although non-Latinx, Latinx and White samples systematically differ in their responses to certain items.47 The HRS sample, like many cohort studies, is relatively more affluent and highly educated than the general US adult population. Individuals from racially minoritized groups, particularly men within these groups, remain drastically underrepresented in population health research because of historical and contemporary mistreatment by the medical community and limitations in effective outreach. Although HRS implemented representative sampling, nonresponse rates may have differed by racial and ethnic identity and experiences of discrimination. If so, this would reduce generalizability, potentially reducing relevance of our findings for older adults experiencing the most discrimination. The relatively small sample size was an additional limitation, leading to imprecise estimates and precluding evaluation of intersectionally defined groups (e.g., Black men). In addition, the sample may reflect a subset of individuals who are generally more interested in research and may be more likely to express interest in AD testing by virtue of agreeing to participate in HRS. This may limit the generalizability of findings and conclusions drawn about those who are not actively engaged in research studies. Furthermore, attitudes may have changed in the decade since this module was fielded. Updated surveys, with larger and more diverse samples, would be invaluable.
Overall, our results suggest that contrary to our hypothesis, past experiences of discrimination, including racial discrimination, are not associated with interest in AD testing. This insight is valuable for clinicians when discussing testing with patients. Past experiences of discrimination may lead patients to eschew particular types of care and hesitate to participate in research. This framing reduces the onus on providers and researchers to achieve equitable access and eliminate barriers to participation for Black and Latinx patients. Our findings are thus key in demonstrating that at least in this question of particular contemporary urgency in AD care and research, differences in uptake of early testing are not likely to be attributable to patient preferences based on experiences of discrimination.
Glossary
- AD
Alzheimer disease
- ADRD
Alzheimer disease and related dementia
- HRS
Health and Retirement Study
- IRB
Institutional Review Board
- RD
risk difference
- UCSF
University of California San Francisco
Appendix. Authors
| Name | Location | Contribution |
| Tanisha G. Hill-Jarrett, PhD | Department of Neurology, Memory and Aging Center, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| Minhyuk Choi, MPH | Department of Epidemiology and Biostatistics, University of California San Francisco | Analysis or interpretation of data |
| Peter Toyokazu Buto, MPH | Department of Epidemiology and Biostatistics, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| Silvia Miramontes, MIDS | Bakar Computational Health Sciences Institute | Drafting/revision of the manuscript for content, including medical writing for content |
| Marilyn D. Thomas, PhD, MPH | Department of Psychiatry and Behavioral Sciences, Weill Institute for Neurosciences, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| Yulin Yang, PhD | Department of Epidemiology and Biostatistics, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| Min Hee Kim, PhD | Institute for Health Policy Studies, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| Kendra D. Sims, PhD | Department of Epidemiology and Biostatistics, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| M. Maria Glymour, ScD | Department of Epidemiology and Biostatistics, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
Study Funding
T.G. Hill-Jarrett: NIA T32AG078115; M.D. Thomas: NIA K99AG076973; M.H. Kim: NIA K99 K99AG078405; K.D. Sims: NIA T32AG049663, S. Miramontes: NIGMS T32GM067547; M.M. Glymour, P.T. Buto, M. Choi: NIA R01AG057869.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Lee S, Kim D, Lee H. Examine race/ethnicity disparities in perception, intention, and screening of dementia in a community setting: scoping review. Int J Environ Res Public Health. 2022;19(14):8865. doi: 10.3390/ijerph19148865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin PJ, Daly AT, Olchanski N, et al. Dementia diagnosis disparities by race and ethnicity. Med Care. 2021;59(8):679-686. doi: 10.1097/MLR.0000000000001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper C, Tandy AR, Balamurali TBS, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193-203. doi: 10.1097/JGP.0b013e3181bf9caf [DOI] [PubMed] [Google Scholar]
- 4.Livney MG, Clark CM, Karlawish JH, et al. Ethnoracial differences in the clinical characteristics of Alzheimer's disease at initial presentation at an urban Alzheimer's disease center. Am J Geriatr Psychiatry. 2011;19(5):430-439. doi: 10.1097/JGP.0b013e3181f7d881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, Alzheimer’s Association. Healthy Brain Initiative, State and Local Public Health Partnerships to Address Dementia: The 2018–2023 Road Map; 2018. cdc.gov/aging/pdf/2018-2023-Road-Map-508.pdf [Google Scholar]
- 6.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280-292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22(1):94-97. doi: 10.1097/WAD.0b013e31815a9dcc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen NC, Leuzy A, Palmqvist S, et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nat Aging. 2020;1(1):114-123. doi: 10.1038/s43587-020-00003-5 [DOI] [PubMed] [Google Scholar]
- 9.Largent EA, Wexler A, Karlawish J. The future is P-tau-anticipating direct-to-consumer Alzheimer disease blood tests. JAMA Neurol. 2021;78(4):379-380. doi: 10.1001/jamaneurol.2020.4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Ortiz F, Turton M, Kac PR, et al. Brain-derived tau: a novel blood-based biomarker for Alzheimer's disease-type neurodegeneration. Brain J Neurol. 2023;146(3):1152-1165. doi: 10.1093/brain/awac407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79(12):1242-1249. doi: 10.1001/jamaneurol.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666-668. [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal J, Halkitis PN. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav Med. 2019;45(2):79-85. doi: 10.1080/08964289.2019.1619511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19(1):4-15. doi: 10.1370/afm.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond WP, Siddiqi AA. Social determinants of medical mistrust among African-American men. In: Social Determinants of Health Among African-American Men. Jossey-Bass/Wiley; 2013:135-160. [Google Scholar]
- 17.Nguyen TT, Vable AM, Glymour MM, Nuru-Jeter A. Trends for reported discrimination in health care in a national sample of older adults with chronic conditions. J Gen Intern Med. 2018;33(3):291-297. doi: 10.1007/s11606-017-4209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19(4):1598-1695. doi: 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 19.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci. 2016;113(16):4296-4301. doi: 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila-Castelar C, Fox-Fuller JT, Guzmán-Vélez E, Schoemaker D, Quiroz YT. A cultural approach to dementia—insights from US Latino and other minoritized groups. Nat Rev Neurol. 2022;18(5):307-314. doi: 10.1038/s41582-022-00630-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;5(1):751-770. doi: 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams DR. Measuring discrimination resource. Psychol. 1997;2(3):335-351. [DOI] [PubMed] [Google Scholar]
- 23.Carter RT, Lau MY, Johnson V, Kirkinis K. Racial discrimination and health outcomes among racial/ethnic minorities: a meta-analytic review. J Multicult Couns Dev. 2017;45(4):232-259. doi: 10.1002/jmcd.12076 [DOI] [Google Scholar]
- 24.Krieger N. Discrimination and health inequities. Int J Health Serv. 2014;44(4):643-710. doi: 10.2190/HS.44.4.b [DOI] [PubMed] [Google Scholar]
- 25.Roberts JS, Barber M, Brown TM, et al. Who seeks genetic susceptibility testing for Alzheimer's disease? Findings from a multisite, randomized clinical trial. Genet Med. 2004;6(4):197-203. doi: 10.1097/01.GIM.0000132688.55591.77 [DOI] [PubMed] [Google Scholar]
- 26.Roberts JS. Anticipating response to predictive genetic testing for Alzheimer’sdisease: a survey of first-degree relatives. Gerontologist. 2000;40(1):43-52. doi: 10.1093/geront/40.1.43 [DOI] [PubMed] [Google Scholar]
- 27.Neumann PJ, Hammitt JK, Mueller C, et al. Public attitudes about genetic testing for Alzheimer's disease. Health Aff (Millwood). 2001;20(5):252-264. doi: 10.1377/hlthaff.20.5.252 [DOI] [PubMed] [Google Scholar]
- 28.Juster FT, Suzman R. An overview of the health and retirement study. J Hum Resour. 1995;30:S7. doi: 10.2307/146277 [DOI] [Google Scholar]
- 29.Williams DR, Yan Yu, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335-351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 30.Smith J, Ryan L, Fisher GG, Sonnega A, Weir D. Psychosocial and Lifestyle Questionnaire 2006-2016: Document Report Core Section LB; 2017. [Google Scholar]
- 31.Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292-323. doi: 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Maurik IS, Vos SJ, Bos I, et al. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol. 2019;18(11):1034-1044. doi: 10.1016/S1474-4422(19)30283-2 [DOI] [PubMed] [Google Scholar]
- 33.Ketchum FB, Chin NA, Grill J, et al. Moving beyond disclosure: stages of care in preclinical Alzheimer's disease biomarker testing. Alzheimers Dement. 2022;18(10):1969-1979. doi: 10.1002/alz.12620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer Disease in a defined population of older persons. Arch Neurol. 1997;54(11):1399-1405. doi: 10.1001/archneur.1997.00550230066019 [DOI] [PubMed] [Google Scholar]
- 35.Wang AY, Hu HY, Ou YN, et al. Socioeconomic status and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 39 prospective studies. J Prev Alzheimers Dis. 2023;10(1):83-94. doi: 10.14283/jpad.2022.81 [DOI] [PubMed] [Google Scholar]
- 36.Meeker KL, Wisch JK, Hudson D, et al. Socioeconomic status mediates racial differences seen using the AT(N) framework. Ann Neurol. 2021;89(2):254-265. doi: 10.1002/ana.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler EM, Bowen CE, Baer M, Froelich L, Wahl HW. Dementia worry: a psychological examination of an unexplored phenomenon. Eur J Ageing. 2012;9(4):275-284. doi: 10.1007/s10433-012-0242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MetLife Foundation. Americans Fear Alzheimer's More than Heart Disease, Diabetes or Stroke, but Few Prepare. 2006.
- 39.MetLife Foundation. What America Thinks. The MetLife Foundation Alzheimer’s Survey. 2011. [Google Scholar]
- 40.Wikler EM, Blendon RJ, Benson JM. Would you want to know? Public attitudes on early diagnostic testing for Alzheimer's disease. Alzheimers Res Ther. 2013;5(5):43. doi: 10.1186/alzrt206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheffrin M, Stijacic Cenzer I, Steinman MA. Desire for predictive testing for Alzheimer's disease and impact on advance care planning: a cross-sectional study. Alzheimers Res Ther. 2016;8(1):55. doi: 10.1186/s13195-016-0223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson CM, Chin NA, Ketchum FB, et al. Predictors of willingness to enroll in hypothetical Alzheimer disease biomarker studies that disclose personal results. Alzheimer Dis Assoc Disord. 2022;36(2):125-132. doi: 10.1097/WAD.0000000000000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer's and dementia: an action plan for solutions. Alzheimers Dement. 2016;12(11):1113-1115. doi: 10.1016/j.jalz.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 44.Kennedy RE, Cutter GR, Wang G, Schneider LS. Challenging assumptions about African American participation in Alzheimer disease trials. Am J Geriatr Psychiatry. 2017;25(10):1150-1159. doi: 10.1016/j.jagp.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vyas MV, Raval PK, Watt JA, Tang-Wai DF. Representation of ethnic groups in dementia trials: systematic review and meta-analysis. J Neurol Sci. 2018;394:107-111. doi: 10.1016/j.jns.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 46.Shaw AR, Perales-Puchalt J, Moore T, et al. Recruitment of older African Americans in Alzheimer's disease clinical trials using a community education approach. J Prev Alzheimers Dis. 2022;9(4):672-678. doi: 10.14283/jpad.2022.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastos JL, Harnois CE. Does the Everyday Discrimination Scale generate meaningful cross-group estimates? A psychometric evaluation. Soc Sci Med. 2020;265:113321. doi: 10.1016/j.socscimed.2020.113321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data are made publicly available through the Health and Retirement Study (hrs.isr.umich.edu/).