Abstract
alpha-Sarcin is a single polypeptide chain protein which exhibits antitumour activity by degrading the larger ribosomal RNA of tumour cells. We describe the interaction of a alpha-sarcin with lipid model systems. The protein specifically interacts with negatively-charged phospholipid vesicles, resulting in protein-lipid complexes which can be isolated by ultracentrifugation in a sucrose gradient. alpha-Sarcin causes aggregation of such vesicles. The extent of this interaction progressively decreases when the molar ratio of phosphatidylcholine increases in acidic vesicles. The kinetics of the vesicle aggregation induced by the protein have been measured. This process is dependent on the ratio of alpha-sarcin present in the protein-lipid system. A saturation plot is observed from phospholipid vesicles-protein titrations. The saturating protein/lipid molar ratio is 1:50. The effect produced by the antitumour protein on the lipid vesicles is dependent on neither the length nor the degree of unsaturation of the phospholipid acyl chain. However, the aggregation is dependent on temperature, being many times higher above the phase transition temperature of the corresponding phospholipid than below it. The effects of pH and ionic strength have also been considered. An increase in the ionic strength does not abolish the protein-lipid interaction. The effect of pH may be related to conformational changes of the protein. Binding experiments reveal a strong interaction between alpha-sarcin and acidic vesicles, with Kd = 0.06 microM. The peptide bonds of the protein are protected against trypsin hydrolysis upon binding to acidic vesicles. The interaction of the protein with phosphatidylglycerol vesicles does not modify the phase transition temperature of the lipid, although it decreases the amplitude of the change of fluorescence anisotropy associated to the co-operative melting of 1,6-diphenyl-1,3,5-hexatriene (DPH)-labelled vesicles. The results are interpreted in terms of the existence of both electrostatic and hydrophobic components for the interaction between phospholipid vesicles and the antitumour protein.
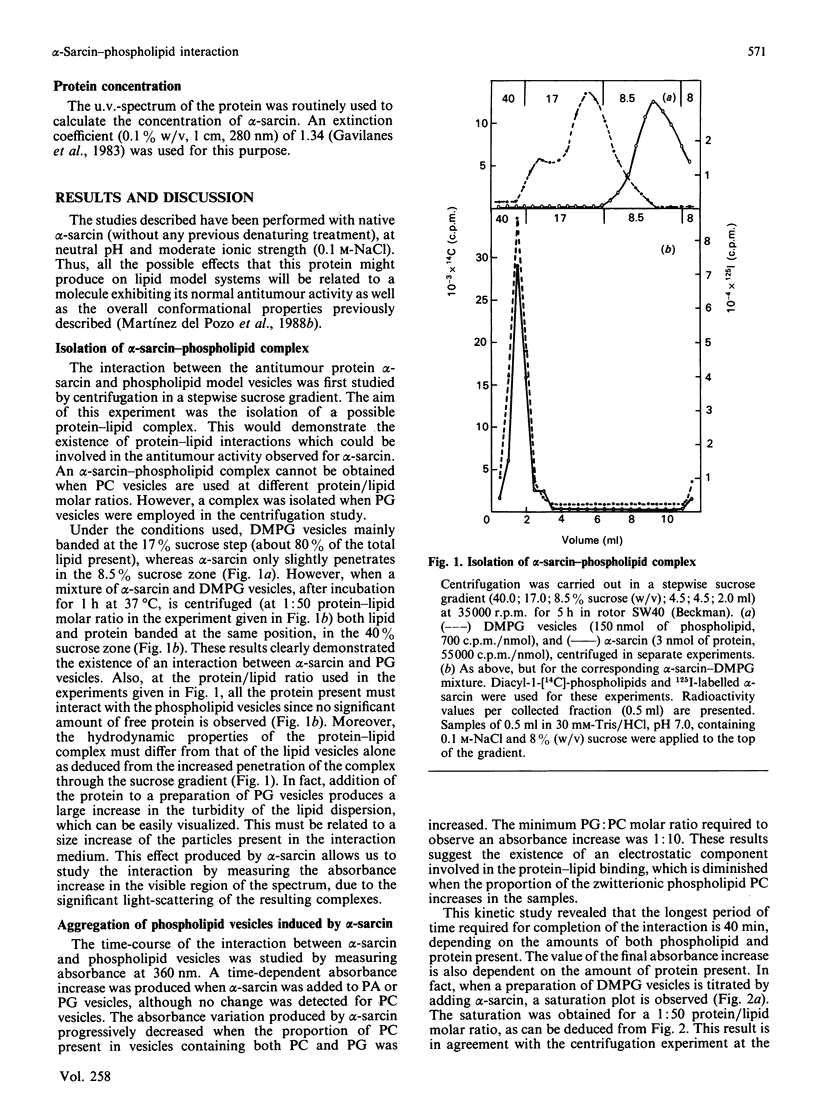
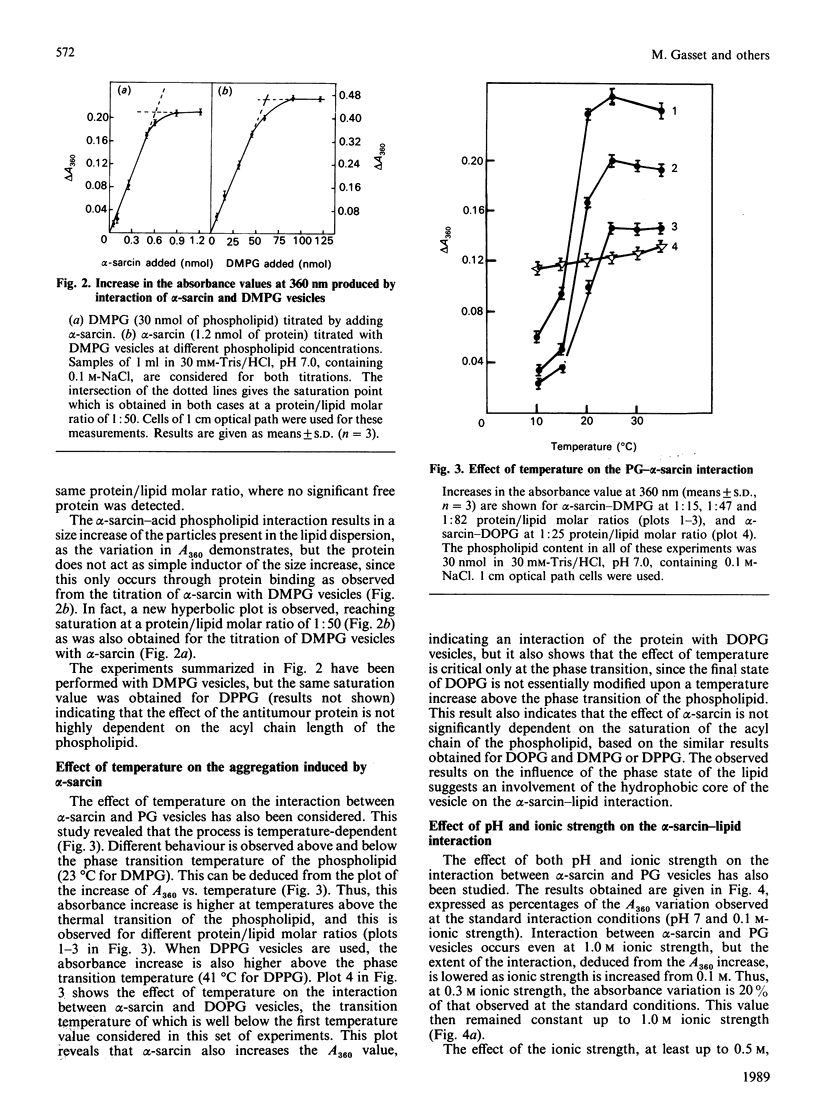
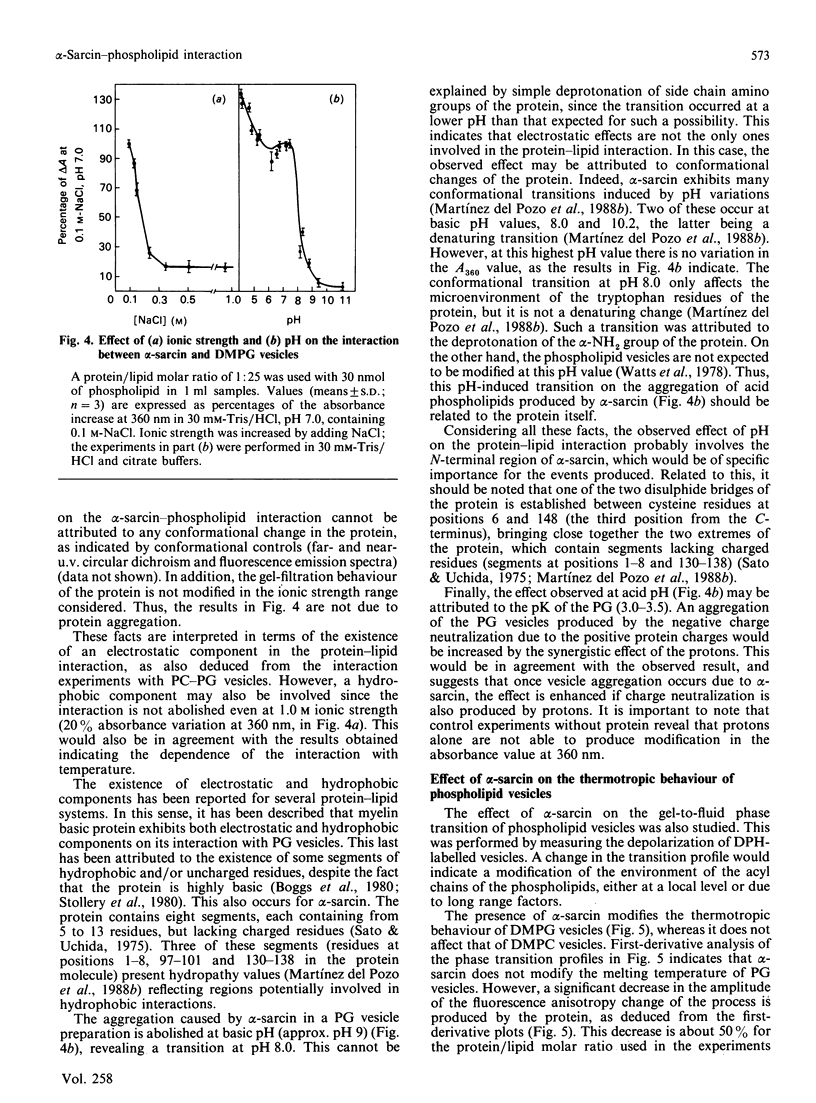
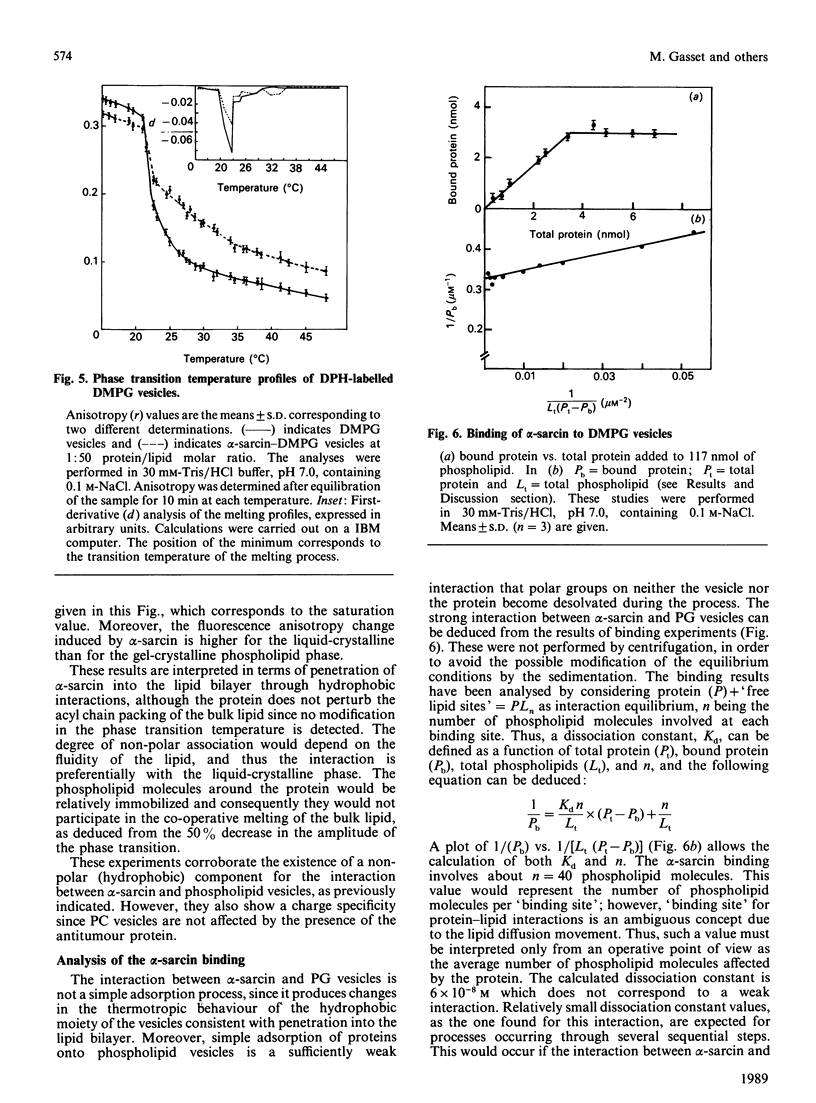
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg A. M., Bougis P. E., Rochat H., Verkleij A. J., de Kruijff B. Penetration of a cardiotoxin into cardiolipin model membranes and its implications on lipid organization. Biochemistry. 1985 Dec 3;24(25):7101–7110. doi: 10.1021/bi00346a013. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Stollery J. G., Moscarello M. A. Effect of lipid environment on the motion of a spin-label covalently bound to myelin basic protein. Biochemistry. 1980 Mar 18;19(6):1226–1234. doi: 10.1021/bi00547a029. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Eytan G. D., Broza R. Role of charge and fluidity in the incorporation of cytochrome oxidase into liposomes. J Biol Chem. 1978 May 10;253(9):3196–3202. [PubMed] [Google Scholar]
- Eytan G. D. Use of liposomes for reconstitution of biological functions. Biochim Biophys Acta. 1982 Oct 20;694(2):185–202. doi: 10.1016/0304-4157(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- Gavilanes J. G., Lizarbe M. A., Municio A. M., Oñaderra M. Fluorescence studies on the lipoprotein complex of the fatty acid synthetase from the insect Ceratitis capitata. Biochemistry. 1981 Sep 29;20(20):5689–5694. doi: 10.1021/bi00523a008. [DOI] [PubMed] [Google Scholar]
- Gavilanes J. G., Lizarbe M. A., Munico A. M., Oñaderra M. Interaction of dipalmitoyl-phosphatidylcholine with calf thymus histone H1. Int J Pept Protein Res. 1985 Aug;26(2):187–194. doi: 10.1111/j.1399-3011.1985.tb03196.x. [DOI] [PubMed] [Google Scholar]
- JENNINGS J. C., OLSON B. H., ROGA V., JUNEK A. J., SCHUURMANS D. M. ALPHA SARCIN, A NEW ANTITUMOR AGENT. II. FERMENTATION AND ANTITUMOR SPECTRUM. Appl Microbiol. 1965 May;13:322–326. doi: 10.1128/am.13.3.322-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez del Pozo A., Oñaderra M., Laynez J., Gavilanes J. G. Interaction of type I collagen with phosphatidylcholine vesicles. Coll Relat Res. 1988 Mar;8(2):133–144. doi: 10.1016/s0174-173x(88)80025-6. [DOI] [PubMed] [Google Scholar]
- Martínez del Pozo A., Gasset M., Oñaderra M., Gavilanes J. G. Conformational study of the antitumor protein alpha-sarcin. Biochim Biophys Acta. 1988 Apr 14;953(3):280–288. doi: 10.1016/0167-4838(88)90036-2. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J., von der Mark K. Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J. 1983;2(1):45–50. doi: 10.1002/j.1460-2075.1983.tb01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesmeyanova M. A. On the possible participation of acid phospholipids in the translocation of secreted proteins through the bacterial cytoplasmic membrane. FEBS Lett. 1982 Jun 7;142(2):189–193. doi: 10.1016/0014-5793(82)80131-2. [DOI] [PubMed] [Google Scholar]
- OLSON B. H., GOERNER G. L. ALPHA SARCIN, A NEW ANTITUMOR AGENT. I. ISOLATION, PURIFICATION, CHEMICAL COMPOSITION, AND THE IDENTITY OF A NEW AMINO ACID. Appl Microbiol. 1965 May;13:314–321. doi: 10.1128/am.13.3.314-321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld A., Sijens P., Verkleij A. J., Kruijff B. Interaction of cytochrome c and its precursor apocytochrome c with various phospholipids. EMBO J. 1983;2(6):907–913. doi: 10.1002/j.1460-2075.1983.tb01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Lopez-Otin C., Barber D., Fernandez-Luna J. L., Gonzalez G., Mendez E. Amino acid sequence homologies in alfa-sarcin, restrictocin and mitogillin. Biochem Biophys Res Commun. 1982 Sep 16;108(1):315–321. doi: 10.1016/0006-291x(82)91868-x. [DOI] [PubMed] [Google Scholar]
- Sato S., Uchida T. The amino acid sequence of ribonuclease U2 from Ustilago sphaerogena. Biochem J. 1975 Feb;145(2):353–360. doi: 10.1042/bj1450353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollery J. G., Boggs J. M., Moscarello M. A. Variable interaction of spin-labeled human myelin basic protein with different acidic lipids. Biochemistry. 1980 Mar 18;19(6):1219–1226. doi: 10.1021/bi00547a028. [DOI] [PubMed] [Google Scholar]
- Takahashi K. The structure and function of ribonuclease T 1 . XVII. Isolation and amino acid sequences of papain and subtilisin peptides from ribonuclease T 1 --the complete covalent structure of ribonuclease T 1 . J Biochem. 1971 Dec;70(6):945–960. doi: 10.1093/oxfordjournals.jbchem.a129724. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Watts A., Harlos K., Maschke W., Marsh D. Control of the structure and fluidity of phosphatidylglycerol bilayers by pH titration. Biochim Biophys Acta. 1978 Jun 16;510(1):63–74. doi: 10.1016/0005-2736(78)90130-x. [DOI] [PubMed] [Google Scholar]
- de Vrije T., de Swart R. L., Dowhan W., Tommassen J., de Kruijff B. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature. 1988 Jul 14;334(6178):173–175. doi: 10.1038/334173a0. [DOI] [PubMed] [Google Scholar]


