Abstract
Observations that intestinal microbes can beneficially impact host physiology have prompted investigations into the therapeutic usage of such microbes in a range of diseases. For example, the human intestinal microbe Limosilactobacillus reuteri strains ATCC PTA 6475 and DSM 17938 are being considered for use for intestinal ailments including colic, infection, and inflammation as well as non-intestinal ailments including osteoporosis, wound healing, and autism spectrum disorder. While many of their beneficial properties are attributed to suppressing inflammatory responses in the gut, we postulated that L. reuteri may also regulate hormones of the gastrointestinal tract to affect physiology within and outside of the gut. To determine if L. reuteri secreted factors impact the secretion of enteric hormones, we treated an engineered jejunal organoid line, NGN3-HIO, which can be induced to be enriched in enteroendocrine cells, with L. reuteri 6475 or 17938 conditioned medium and performed transcriptomics. Our data suggest that these L. reuteri strains affect the transcription of many gut hormones, including vasopressin and luteinizing hormone subunit beta, which have not been previously recognized as being produced in the gut epithelium. Moreover, we find that these hormones appear to be produced in enterocytes, in contrast to canonical gut hormones which are produced in enteroendocrine cells. Finally, we show that L. reuteri conditioned media promotes the secretion of several enteric hormones including serotonin, GIP, PYY, vasopressin, and luteinizing hormone subunit beta. These results support L. reuteri affecting host physiology through intestinal hormone secretion, thereby expanding our understanding of the mechanistic actions of this microbe.
Keywords: enteroendocrine, enterocyte, hormone, small intestine, Lactobacillus, vasopressin, luteinizing hormone, GIP, PYY, adipolin, kisspeptin
Introduction
The use of commensal microbes in the treatment of disease has the potential to herald in a new era of microbial-based therapeutics. The human associated Limosilactobacillus reuteri is one such microbe considered for development as a therapeutic: it has been shown to improve symptoms of infant colic1, osteoporosis2, and inflammatory diseases3–6, and is being considered for its role in alleviating asocial behavior associated with autism spectrum disorder7–11. How L. reuteri mediates these effects is not fully understood. Moreover, several different L. reuteri strains are currently in use, highlighting the importance of studying strain variation in understanding therapeutic efficacy.
Two of the commonly employed strains that are currently marketed as probiotics are L. reuteri ATCC PTA 6475 and L. reuteri DSM 17938. While both were originally derived from human breast milk, these strains are phylogenetically and functionally distinct. L. reuteri 6475, belongs to L. reuteri clade II, while L. reuteri 17938 (derived from strain ATCC 5573012) belongs to L. reuteri clade VI13. L. reuteri 17938 (or its parent L. reuteri 55730) has been demonstrated to reduce infant colic1, assist in feeding tolerance in preterm infants14, improve intestinal motility in preterm14 and term infants15, and improve cytokine ratios in children with apoptotic dermatitis16. L reuteri 6475 has been shown to have potential in relieving inflammatory conditions through TNF suppression, which may be linked to its capacity to reduce osteoporosis2,17–23. L. reuteri 6475 has also been demonstrated efficacious in promoting wound healing24,25, restoring normal social behavior in mouse models of autism7–9,11 (which L. reuteri 17938 has been shown unable to do so in mice), and improving male reproductive health in mice26. These two strains are similar in their ability to produce the antimicrobial reuterin and the vitamins pseudo B12 and B9 (folate)27 and to produce proteins for host mucus adherence28. L. reuteri 6475 can also produce histamine while L. reuteri 17938 cannot13. This histamine production is implied in L. reuteri 6475’s suppression of the inflammatory signal tumor necrosis factor (TNF)27. L. reuteri 17938 has also been demonstrated to liberate adenosine from AMP, which may be involved in its function to reduce autoimmunity in Treg deficiency disorders by enhancing CD73+CD8+T cells29.
While many of L. reuteri’s functions are thought to be due to interactions with immune cells, L. reuteri itself or its secreted products has the capacity to influence host physiology through a wide range of cell types. Particularly in the small intestine, where the mucus layer is thin, L. reuteri may have ample opportunities to interact with the host epithelial cells. Given, the diverse roles of L. reuteri in gut motility, on inflammatory processes, and on the gut-brain axis led us to consider whether some of L. reuteri’s interactions with the host are mediated through enteroendocrine cells.
Enteroendocrine cells are secretory cells in the intestine specialized for the secretion of hormones. Enteroendocrine cells sense nutrients like sugars, peptides, and fatty acids in the intestinal lumen through G-protein coupled receptors and utilize ion (sodium, hydrogen, calcium) transporters to bring nutrients into the cell30. On apical entry or basolateral exit from enteroendocrine cells, these nutrients can trigger hormone receptors and lead to the release of hormones from the apical or basolateral side of the cell31. Enteroendocrine cells also respond to microbial stimulus through toll-like receptors to release cytokines and subsequently affect inflammatory responses30. As well, released gut hormones can directly and indirectly influence pro- and anti-inflammatory immune cell populations through a variety of mechanisms30. Finally, enteroendocrine cells and a few specific hormones are associated with the integrity of the intestinal barrier30.
Enteroendocrine cells, however, comprise ~1% of gut epithelial cells, thereby making study of these cells difficult in vivo and in non-transformed tissue lines. To overcome this limitation, we recently developed a human enteroendocrine-enriched jejunal organoid line32. Through induction of the developmental regulator of enteroendocrine cells, NGN3, we can increase the number of enteroendocrine cells to ~40% in this adult cell stem derived human jejunal organoid line at the expense of enterocytes32.
Here, we utilized these NGN3 human intestinal organoids (HIOs) to characterize how L. reuteri secreted products impact enteroendocrine cells. By performing RNA-Seq on uninduced organoids and induced, enteroendocrine-enriched organoids, we observe that L. reuteri affects the transcription of genes involved in hormone secretion, nutrient sensing, cell adhesion, mucus production, immune/stress response, and cell fate. Among the impacted hormones are enterocyte-derived hormones, not previously characterized in the intestinal epithelium. For several of the impacted hormones, we additionally demonstrate that L. reuteri promotes the secretion of these hormones from HIOs or from ex vivo human intestinal tissue. In general, we observe similar effects of L. reuteri strains 6475 and 17938 on epithelial cells but with L. reuteri 6475 having a greater magnitude of effect on transcription. These results suggest specific mechanisms by which L. reuteri mediates its beneficial effects with a magnified look at how L. reuteri interacts with enteric hormones.
Methods
Preparation of bacterial conditioned media
L. reuteri strains ATCC PTA 6475 and DSM 17938 were provided by BioGaia (Sweden). A single colony of L. reuteri 6475 or 17938 from an MRS agar plate was inoculated into 10 mL of MRS broth and incubated in a tightly closed conical tube in a 37°C water bath or incubator. After 15 hours of incubation, the L. reuteri culture was diluted to an OD600 of 0.1 into 25 to 40 mL of pre-warmed LDM46 and placed into a 37°C water bath to incubate until reaching an OD600 of 0.5–0.6. Next, cells were pelleted by centrifugation and the resulting supernatant was transferred to a new conical tube. The pH of the supernatant was measured by applying 2 μL of the supernatant onto pH paper (range 6.0 – 8.0, Fisherbrand, Pittsburgh, PA, USA) and adjusted to 7.0 using 10 M sodium hydroxide solution. Neutralized conditioned media and LDM4 media control were filter sterilized (0.22μm PVDF membrane, Steriflip, EMD Millipore, Burlington, MA), aliquoted, frozen at −80°C overnight, and then lyophilized. Lyophilized conditioned media were stored at −20°C until use.
Propagation of organoids and organoid media
J2 NGN3 organoids were propagated in 3D in CMGF+ media32 + 10 μmol Y-27632 Rock inhibitor + 200 μg/ml geneticin as previously described33. NGN3-HIOs were then seeded onto 24-well transwells and differentiated in the presence of differentiation media32 with (induced) or without (uninduced) 1 μg/ml doxycycline.
Transwell assay
For use on organoids, lyophilized conditioned media were resuspended in an equal volume of organoid differentiation media. The existing differentiation media on the apical side of the transwells were removed and replaced with 100 μL differentiation media supplemented with lyophilized conditioned media or media control. Transwells were incubated for 3 hours at 37°C with 5% CO2. Following, apical and basolateral supernatants were removed and stored at −20°C in a 96 well plate to be used later in a hormone secretion assay. The transwell membrane was removed from the support surface and placed in TRIzol solution (Invitrogen, Waltham, MA, USA). Following a chloroform extraction, the aqueous phase containing total RNA was immediately extracted using a Qiagen RNeasy kit (Qiagen, Germantown, MD, USA).
RNA-Seq
Paired-end Illumina sequencing libraries were prepared by Novogene (Sacramento, CA, USA). Briefly, total RNA was enriched for Eukaryote mRNA. mRNA was fragmented to an average insert size of 250 to 300 bp, and cDNA was prepared using the standard NEB library construction method. The library was 150 bp paired-end sequenced on a NovaSeq 6000. Basecalling was performed using CASAVA v1.834. Reads were filtered as follows: reads containing adaptors were removed, reads with more than 10% N reads were removed, and reads where > 50% of the bases have Qscore <= 5 were removed.
Sequenced reads were aligned to the human genome hg19 using Star (v2.5)35 using the Maximal Mappable Prefix for junction reads and with mismatch = 2. Read counts per gene were tabulated with HTSeq v0.6.136. The gene count table provided by Novogene was further processed using a pipeline derived from iDEP version 0.8237. Genes were filtered to keep those with at least 1 count per million in 5 samples, thereby retaining 15,369 genes.
For multidimensional scaling, rlog transformed data were visualized using a t-distribution to estimate the hypothetical spread of the data. The contribution of induction and L. reuteri treatment to the variation in data were modeled using a permutational multivariate analysis of variance (PERMANOVA) of the form: Euclidean distance matrix ~ induction + treatment + induction * treatment using the adnois function in vegan (v2.5–5)38.
For correlation analyses, rlog-transformed values were used. Lowly expressed genes belonging to the bottom quartile were removed. Correlations among samples were computed using a Pearson correlation. Correlations were visualized using the ComplexHeatmap package (v2.3.1)39, with rows and columns clustered by a Euclidean distance metric and using complete linkage clustering for both. Within and between sample distances were plotted using the ggboxplot function in ggpubr (v0.2.4)40. Significance among distances was calculated by a t-test with a multiple testing correction using Holm’s method41. Difference between means (circle size) and adjusted p-values (circle color) were visualized as a correlogram using ComplexHeatmap package (v2.3.1)39.
For identification of differentially expressed genes, gene counts were modeled as genecount ~ treatment-induction + organoid_batch in DESeq242 V1.22.2 using a Wald test with p values corrected using the Benjamini-Hochberg procedure43 with an FDR cutoff of 0.1 and a fold change cutoff of 2. DESeq2 models the underlying variation using a negative binomial distribution. LDM4 (media alone) and uninduced (not enteroendocrine enriched) were used as reference levels.
Functional analyses
Ensembl IDs release 95 were converted to Ensembl IDs release 98 before analyzing for statistical enrichment of gene functions using the Ensembl ID converter44. Annotations for PANTHER GO-Slim Biological Process, PANTHER GO-Slim Molecular Function, PANTHER GO-Slim Cellular Component, PANTHER Protein Class, Panther Pathways, and Reactome45,46, were performed in PANTHER47, using a binomial test, and a false discovery cutoff of 0.05. Genes belonging to enriched (not depleted) functional categories defined by PANTHER47 were searched in GeneCards48 and annotated into one of the following broad groups: Cell fate/growth, Hormone secretion, Immune response, Membrane component, Mucus, Nutrient metabolism/response, Signaling, or Metal/stress response. Enrichments of these groups within Kmeans determined clusters (see below for heatmap visualization) were determined using a hypergeometric distribution, and all p-values across groups and clusters were corrected en masse using the Benjamini-Hochberg43 method, whereby FDR values less than 0.1 were considered significant.
Data visualization
For multidimensional scaling, clustering, and heatmap visualization, read counts were transformed using the rlog function from DESeq2 V1.22.242. For displaying the gene expression data as a heatmap, the rlog transformed data were batch corrected using the removeBatchEffect command in the limma package49 and the data were centered and scaled using the scale function in base R50. Heatmaps were visualized using the ComplexHeatmap package39, with rows (genes) clustered with the Pearson distance metric and columns (samples) clustered with the Euclidean distance metric, using complete linkage clustering for both. The number of clusters to group the displayed genes was determined using the Kmeans function in base R50, with visualization of the total sum of squares as an elbow plot and average silhouettes in a silhouette plot. The number of clusters to group the samples (columns) was selected solely for enhancing visualization. For barplots of individual gene expression values, read counts were transformed using the GeTMM method51 and converted to counts per million using calcNormFactors and cpm commands in edgeR52. Displayed log2 fold changes were derived from DESeq2 modeled data. In this method, the log fold changes are shrunken to prevent overestimation of fold changes for genes with low counts and/or high dispersion. Enterocyte and enteroendocrine cell markers were referenced from Haber and colleagues53.
Gene annotations
Annotations for select hormone-related genes were taken from GeneCards48 (www.genecards.org) and from the literature: AGT54,55, ARHGEF2556, CCK57–59, GAST57,60,61, GHRL and GHRLOS62–64, GIP60, MLN57,60, NPW65–67, NPY68,69, SST70–72, DRD173, NRG474–76, NTSR160,77, TAC368,78,79, AVP80–85, C1QTNF1286,87, LHB88,89, NTS60,77, OXT8,84,85,90,91, SCTR92, PAQR593, P2RY194, RARB95. Annotations for select immune and stress response genes were taken from GeneCards48 (www.genecards.org).
Human tissue
Human intestinal tissue was acquired from the organ donation group LifeGift within the Texas Medical Center. All organ donors were adults not presenting with any known gastrointestinal disease, surgery, or trauma. Individuals positive for hepatitis B or C, HIV, or COVID were excluded. Tissue was delivered to lab within ~4 hours of the patient initiating organ harvest and within ~1 hour of harvest of the gastrointestinal tract.
Hormone secretion
To measure secreted hormones from the treated organoids, supernatants from the apical (or basolateral, where noted) side of the transwells were assessed using the Luminex MILLIPLEX Human Metabolic Hormone kit (EMD Millipore, USA) or using a serotonin ELISA (SER39-K01, Eagle Biosciences, USA). For measuring hormones secreted from whole human tissue, approximately 2 cm by 2 to 3 cm pieces of human tissue were incubated in 5 mLs of L. reuteri conditioned media or media control in 6 well plates for 3 hours at 5% CO2. AVP was measured with the Arg8-Vasopressin ELISA kit (ADI-900–017A, Enzo, USA), LHB with the Luteinizing Hormone (hLH) ELISA Assay kit (HLH31-K01, Eagle Biosciences, USA), adipolin with the Human CTRP12 ELISA kit (SK00392–06, Aviscera Bioscience, USA), and kisspeptin with the Human Kisspeptin ELISA kit (ab288589, Abcam, USA). For organoids, statistical significance was determined using a one-way ANOVA followed by a Dunnett’s test with the LDM4 treatment used as the control. For human tissue, data were modeled with linear mixed models with the human patient included as a random variable using the lmer function of the lme496 package with REML = FALSE and the control optimizer = “bobyqa”. Following, statistical significance was determined using the emmeans function97 with a Benjamini-Hochberg multiple testing correction.
Single cell RNA-Sequencing analysis
Single cell RNA-Sequencing (scRNA-Seq) analysis of the Human Gut Atlas (https://www.gutcellatlas.org/, adult epithelium, jejunum) was performed as previously described33. Briefly, scRNA-Seq data from the adult jejunum were analyzed using the Seurat package in R (v 5.0.3). After data normalization, data clustering, and UMAP generation, genes of interest were plotted using the FeaturePlot function.
Immunofluorescence
Adipolin was visualized on human jejunal tissue from organ donors as previously described33 using the antibody (NBP1–90700, Novus Biologicals) diluted to between 1:20 to 1:50 and detected with Rhodamine Red™-X (AB_2338028, Jackson ImmunoResearch) diluted to 1:200. An E-caderin conjugated antibody (1:50, 560062, BD Pharmingen) and DAPI (1:10, NucBlue™ Fixed Cell Stain ReadyProbes™ reagent, R37606, Invitrogen) were applied simultaneously with Rhodamine Red™-X.
Data availability
RNA-Seq reads are available at NCBI GEO at https://www.ncbi.nlm.nih.gov/geo/ accession number GSE138350 and GSE268681. Scripts for plots and data are available at https://github.com/sdirienzi/Lreuteri_HIORNASeq. An interactive ShinyApp displaying the RNASeq data can be found here: https://sdirienzi.shinyapps.io/LreuHIORNASeq/.
Results
NGN3-HIOs facilitate study of L. reuteri’s interactions with the enteroendocrine system
To determine how L. reuteri strains 6475 and 17938 affect the intestinal epithelium, we designed an RNA-Seq experiment using human intestinal organoids (HIOs) treated with pH neutralized conditioned media produced by these strains in log phase (Figure 1A). The media thereby represent any products released by the L. reuteri strains into their growth media. The specific HIOs we utilized originated from adult jejunal stem cells and have been engineered for the inducible expression of the transcription factor NGN3. NGN3 induction results in HIOs enriched in enteroendocrine cells with a decrease in the relative abundance of enterocytes32. With this NGN3-HIO line we can measure the effects of the L. reuteri strains on induced NGN3-HIOs enriched in enteroendocrine cells and on uninduced NGN3-HIOs largely comprised of enterocytes.
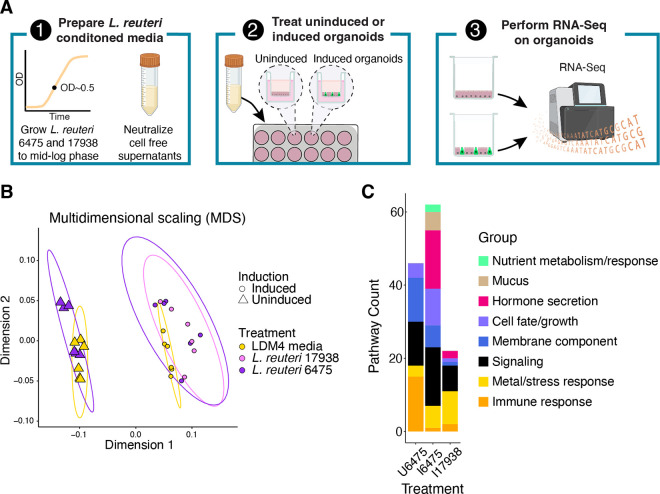
Figure 1.
Induced and uninduced NGN3-HIOs differentially respond to L. reuteri treatment. A) Overview of RNA-Seq experiment. First, L. reuteri conditioned media was prepared by growing L. reuteri 6475 and 17938 in LDM4 to mid-log phase. The bacterial cells were spun out, the resulting conditioned media brought to neutral pH, and then filtered through a 0.22 μm filter. The conditioned media were then lyophilized and resuspended in HIO differentiation media. These treatments were then placed into uninduced or induced NGN3-HIOs in transwells for three hours. Third, the organoid cells were harvested, and isolated RNA was sent for RNA-Seq. Created with BioRender.com. B) Principal coordinate analysis of transcriptomic data from NGN3-HIOs induced or not induced and treated with L. reuteri 6475, 17938, or LDM4 media control. Ellipses for illustration purposes are modeled from the data following a t-distribution. C) Enriched functional categories of differentially expressed genes in L. reuteri treatments over media alone. U6475 is L. reuteri 6475 vs media control in uninduced NGN3-HIOs. I6475 is L. reuteri 6475 vs media control in induced NGN3-HIOs. I17938 is L. reuteri 17938 vs media control in induced NGN3-HIOs. Some functional groups are listed as belonging to two categories (see Supplemental Table 3 for further details).
We tested L. reuteri 6475 on uninduced NGN3-HIOs (~90% enterocytes, <2% enteroendocrine cells)32 and L. reuteri 6475 and 17938 on enteroendocrine-enriched (induced) NGN3-HIOs (~50% enterocytes, ~40% enteroendocrine cells)32. L. reuteri 17938 was not tested on uninduced HIOs. RNA-Seq of the organoids produced an average of 16.1 million reads per library (Table 1, Supplemental Table 1). To confirm that induced NGN3-HIOs were enriched in enteroendocrine cells and depleted in enterocytes compared to uninduced NGN3-HIOs, we checked the expression level of known enterocyte and enteroendocrine cell markers53 (Figures S1 and S2). The expression levels of genes followed the expected patterns with enterocyte markers being downregulated and enteroendocrine markers increasing with NGN3 induction (Figures S1 and S2).
Table 1:
Summary of RNA-Seq libraries.
| HIO type | Treatment | Number of HIO experiments | Number of replicate HIOs within an experiment | Total number of RNA-Seq libraries | Average read count |
|---|---|---|---|---|---|
| Uninduced | LDM4 | 2 | 3 | 6 | 18968834.33 |
| Uninduced | L. reuteri 6475 | 2 | 3 | 6 | 15465905.00 |
| Enteroendocrine-enriched | LDM4 | 2 | 3 | 6 | 15972039.33 |
| Enteroendocrine-enriched | L. reuteri 6475 | 2 | 3 | 6 | 14287207.00 |
| Enteroendocrine-enriched | L. reuteri 17938 | 2 | 3 | 6 | 15720129.33 |
Read counts shown are post filtering and alignment to the human genome (See Methods). See Supplemental Table 1 for further details.
To globally assess whether the HIOs were impacted by the L. reuteri conditioned media, we performed an unsupervised analysis using dimensionality reduction with multidimensional scaling (MDS) produced from a Euclidean distance matrix of the gene expression data. As expected, the MDS plot illustrated that the data could be separated in dimension 1 by whether the HIOs were induced for NGN3 expression or not, indicating NGN3 induction was likely the greatest contributor to the variation in global gene expression (Figure 1B). To quantify the contribution of induction as well as the contributions of L. reuteri treatments and biological replication, we performed a PERMANOVA on the Euclidean distance matrix. Our PERMANOVA model reported that NGN3 induction explains 70.8% of the variation (pseudo-F = 120.763, p = 0.001), biological replication 9.2% of the variation (15.685, p = 0.001), L. reuteri treatment 4.4% of the variation (pseudo-F = 3.768; p = 0.011), and that the interaction of treatment and induction was not significant (1.5% of the variation; pseudo-F = 2.578; p = 0.082). Similar results were obtained using the Jaccard similarity index. These results indicated that most of the variation in data resulted from NGN3 induction, and that the addition of L. reuteri 6475 or 17938 had a relatively smaller but still significant effect on HIO gene expression.
To gain further insight into the variation in gene expression in our data, we investigated gene expression correlations among pairwise comparisons of samples. We observed that induced HIOs treated with either L. reuteri strain were significantly less correlated from L. reuteri 6475 vs media control on uninduced HIOs (Supplemental Figure 3A, Supplemental Figure 3B). We also observed that the correlations between induced HIOs treated with L. reuteri 6475 vs their media controls compared to those treated with L. reuteri 17938 vs their media controls were similar (p=0.09), although the mean correlation for induced HIOs treated with L. reuteri 6475 vs their media controls was lower (Supplemental Figure 3A, B). Together, these results further support that both L. reuteri strains had a significant effect on HIO gene expression when the HIOs were induced and suggest that the L. reuteri strains similarly affected gene expression.
L. reuteri strains 6475 and 17938 impact the expression of hormone, nutrient, mucus, metal/stress response, and immune-related genes in native and/or enteroendocrine-enriched HIOs.
We next sought to determine the genes impacted by L. reuteri strains 6475 and 17938 in the induced and uninduced NGN3 HIOs. We identified differentially expressed genes (DEGs) between these two strains and across the induction state of the HIOs. Specifically, we compared the effect of L. reuteri 6475 in the uninduced and induced states compared to their media controls and L. reuteri 17938 in the induced state to its media control. We find a similar number of genes impacted by L. reuteri 6475 in induced and uninduced HIOs, but fewer DEGs by L. reuteri 17938 in induced HIOs (Table 2, Supplemental Table 2). While at first glance, this may suggest L. reuteri 6475 affects HIO differently than 17938, only 12 genes were differentially expressed between L. reuteri 6475 and 17938 in induced HIOs (Table 2; Supplemental Figure 3C). On investigating the gene expression data, we observed that L. reuteri 17938 largely affects gene expression in the same direction as 6475, but that the fold change in gene expression for 17938 failed to pass our significance thresholds. These results reinforce the results of our correlation analysis (Supplemental Figure 3) suggesting that though L. reuteri 6475 had a more potent effect on transcriptional change in our induced HIOs in this experimental setup, the two strains had largely similar effects on gene transcription.
Table 2:
Summary of genes differentially regulated between L. reuteri strains 6475 and 17938 in induced and uninduced HIOs.
| Comparison | Up-regulated | Down-regulated |
|---|---|---|
| U6475-ULDM4 | 359 | 148 |
| I6475-ILDM4 | 307 | 189 |
| I17938-ILDM4 | 66 | 330 |
| I6475-I17938 | 1 | 331 |
Groups are labeled as “U” for uninduced, “I” for induced, “6475” for treatment with L. reuteri 6475 conditioned medium, “17938” for treatment with L. reuteri 17938 conditioned medium, and “LDM4” for treatment with bacterial growth medium.
To determine how these transcriptional changes might functionally affect the HIOs, we looked for functional enrichments in the DEGs. Using the PANTHER classification system47 and the Reactome annotated pathways45,46, we identified enriched functional annotations within the sets of DEGs. Broadly across all datasets, the L. reuteri DEGs were enriched in functions regarding response to the environment. These functions included those for nutrient, stress, metal, and immune response, cell fate/growth, membrane components, and signal transduction (Figure 1C & Supplemental Table 3). As anticipated, the induced HIOs treated with either L. reuteri strain were also enriched in genes for hormone secretion (Figure 1C & Supplemental Table 3). The induced cells treated with L. reuteri 6475 were additionally enriched for genes relating to mucus.
To further investigate and understand the DEGs and their regulation, we annotated these genes within the functional groups and looked for similar expression patterns and functions (Supplemental Table 3). We were able to group the genes into 8 groups using Kmeans clustering (Supplemental Figure 4). These clusters represent genes with similar transcriptional responses to induction and the presence of L. reuteri and therefore may share similar regulatory mechanisms. For instance, genes within a cluster may share a transcription factor or be localized within the same cell type. As cell types within the small intestine have partially non-overlapping functions98, this scenario would promote clusters being enriched in one or two closely related functions.
Indeed, we observed this to be the case (Table 3): clusters were either enriched in one or two related functions or were not enriched in any function. Clusters 1 and 5 were enriched in genes involved in hormone secretion; cluster 2 in cell adhesion; cluster 3 in stress/immune response; cluster 6 in nutrient response; and clusters 7 and 8 in mucus genes. Therefore, the clusters generated by our heatmap are consistent with gene clusters of related functionalities, perhaps from genes expressed in the same or similar cell type.
Table 3:
Functional enrichments within clusters of similarly expressed DEGs.
| Cluster | U6475-ULMD4 | I6475-ILDM4 | I17938-ILDM4 | I6475-U6475 | ILDM4-ULDM4 | Cell adhesion | Cell fate | Hormone secretion | Mucus | Nutrient response | Signaling | Stress/Immune response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | + | + | 0.454 | 0.793 | 0.094 * | NA | 0.542 | 0.001 ** | 1 | |
| 2 | − | − | − | + | + | 0.064 * | 0.879 | 0.879 | 0.231 | 0.542 | 0.542 | 0.454 |
| 3 | + | + | + | + | + | NA | 0.82 | 0.454 | NA | 0.82 | 0.879 | 0.021 * |
| 4 | + | − | NA | 0.106 | NA | NA | 0.542 | 0.399 | 0.655 | |||
| 5 | + | + | + | − | − | NA | 0.542 | 0.064 * | NA | 0.283 | NA | 0.454 |
| 6 | − | − | − | NA | 0.454 | 0.769 | NA | 0.037 * | NA | 0.399 | ||
| 7 | − | − | − | − | 0.231 | 0.454 | 0.879 | 0.064 * | 0.879 | 0.542 | 0.542 | |
| 8 | − | NA | 0.283 | 0.769 | 0.086 * | NA | 0.772 | 0.399 |
Clusters are listed as in Supplemental Figure 4. Columns U6475-ULDM4 through ILDM4-ULDM4 summarize whether genes within that cluster are predominantly up (+) or down (−) regulated for the given comparison. FDR corrected significance values for functional groups:
q<0.1
q<0.01
q<0.001.
L. reuteri impacts on immune and stress response
To see if our data are consistent with known functions of L. reuteri on the intestinal epithelium, we first investigated the immune and stress response DEGs. We observed many immune-related genes were downregulated and a few metal and stress response genes were upregulated by L. reuteri 6475 (Supplemental Figure 5A). Tumor necrosis factor (TNF), which L. reuteri 6475 has been previously observed to downregulate17 and suppress3, was not expressed in our HIOs; however, TNFSF15, which is induced by TNF and activates NF-kappaB99, was decreased in induced HIOs treated with L. reuteri 6475. Consistent with L. reuteri 6475 mediated suppression of NF-kappaB and inflammatory responses17, several chemokines were downregulated by L. reuteri 6475: IL-8 (CXCL8), CCL2 (MCP-1), CXCL2, CX3CL1, and CXCL3. Secreted MCP-1, we observe, was also repressed by both L. reuteri strains only on induced NGN3-HIOs (Supplemental Figure 5B), suggesting a role of enteroendocrine cells in downregulating inflammatory responses. TLR4 which senses stimuli and upregulates inflammatory responses100 was also downregulated by L. reuteri 6475. L. reuteri 6475 additionally upregulated interleukin 18 binding protein (IL18BP), which is an inhibitor of the proinflammatory IL-18101. Defensin-6, interferon epsilon, several metallothionein genes, and aquaporin-1 and −7, which respond to environment changes, were upregulated by L. reuteri 6475. These data are consistent with reports of L. reuteri 6475 having anti-inflammatory, immune modulatory, and stress response effects on the gut epithelium. L. reuteri 17938 had a less pronounced effect on immune and stress response genes. None of the chemokine or aquaporin genes were significantly impacted and only about half of the metallothioneins were differentially regulated in response to L. reuteri 17938. As mentioned previously, these results largely appear to be the result of L. reuteri 17938 impacting gene expression in the same direction but not the same magnitude as 6475 in our experiment.
L. reuteri affects the transcription and secretion of enteroendocrine cell hormones
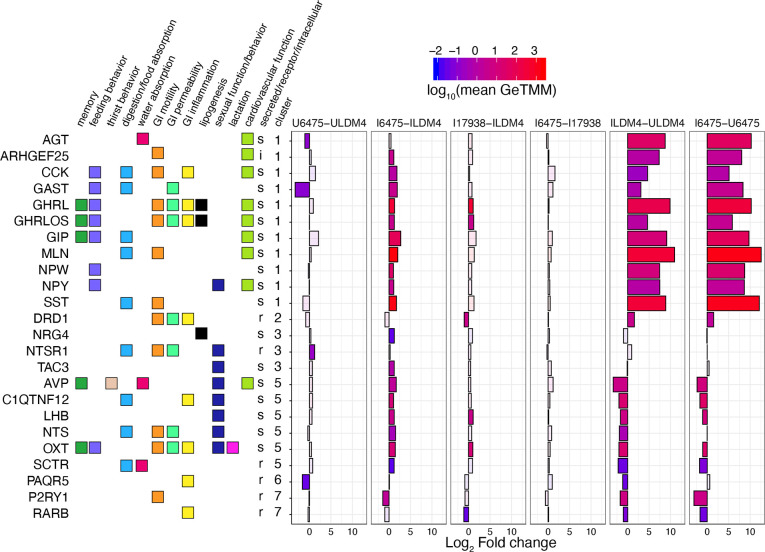
We next focused on clusters 1 and 5 for their enrichment of hormone genes (Figure 2). Cluster 1 appears as we would expect for canonical gut hormones derived from enteroendocrine cells: the genes in cluster 1 increased in expression with NGN3 induction. These genes included those for the hormones angiotensinogen (AGT), cholecystokinin (CCK), gastrin (GAST), ghrelin (GHRL and GHRLOS), gastric inhibitory polypeptide aka glucose dependent insulinotropic polypeptide (GIP), motilin (MLN), neuropeptide W (NPW), neuropeptide Y (NPY), and somatostatin (SST). With the exception AGT, all genes were significantly upregulated by L. reuteri 6475. Only GHRL and GHRLOS were significantly upregulated by L. reuteri 17938.
Figure 2:
Hormone genes differentially expressed by L. reuteri. DEGs annotated as having hormonal function are shown. The genes are annotated with their function, whether they are secreted, a receptor, or intercellular, and what cluster they belong to as in Supplemental Figure S4. The graph shows the log2 fold change expression of the gene for the indicated comparison. The bars are colored using the log10 scaled mean GeTMM counts to illustrate how abundantly expressed the gene is. Transparent overlays are used on genes not differentially expressed for the given comparison. Comparisons shown: U6475-ULDM4, L. reuteri 6475 on uninduced HIOs compared to LDM4 media control; I6475-ILDM4, L. reuteri 6475 on induced HIOs compared to LDM4 media control; I17938-ILDM4, L. reuteri 17938 on induced HIOs compared to LDM4 media control; I6475-I17938 L. reuteri 6475 compared to L. reuteri 17938 on induced HIOs; ILDM4-ULDM4, LDM4 media control on induced versus uninduced HIOs; I6475-U6475, L. reuteri 6475 on induced versus uninduced HIOs. For each, positive fold changes indicate genes upregulated by the condition listed first.
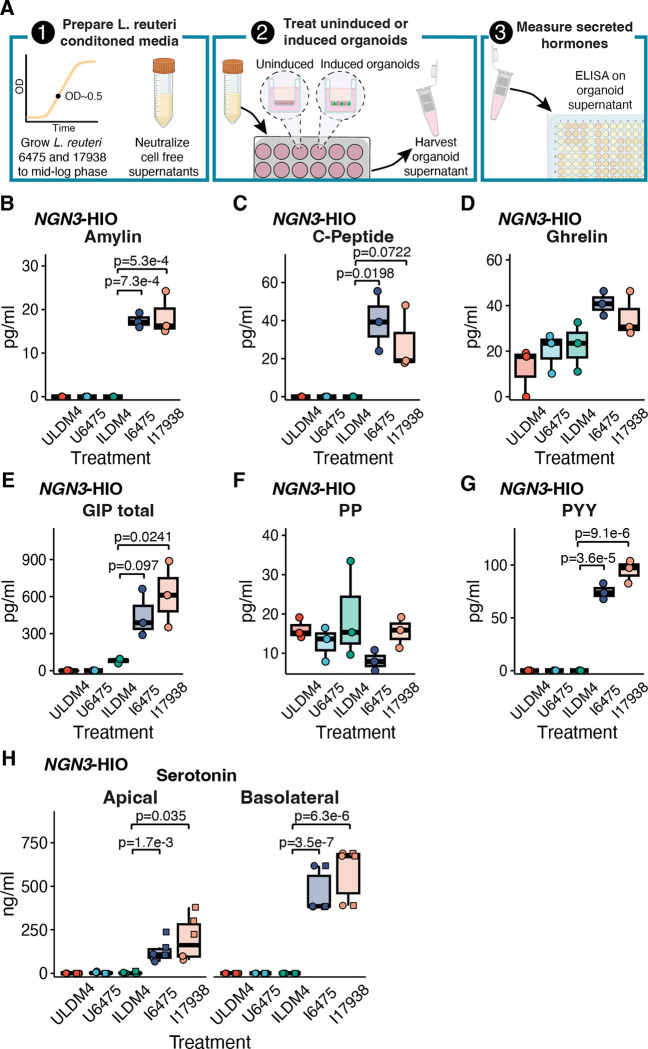
To determine if some of these gene expression differences might lead to differences in hormone secretion, we tested the organoid supernatant that had been collected following the application of L. reuteri 6475 and 17938 conditioned media to uninduced and induced NGN3-HIOs. The harvested supernatants coming off the organoids were run on a Luminex panel consisting of metabolic-related hormones (see Methods) (Figure 3A). From this panel, we were able to obtain measurable values of amylin, C-peptide, ghrelin, GIP (total), pancreatic polypeptide (PP), and peptide YY (PYY) (Figure 3B–G). For amylin and PYY, both L. reuteri strains significantly increased secretion of these hormones from induced NGN3-HIOs (Figure 3B, G). The secretion of GIP was enhanced significantly (at p<0.05) by L. reuteri 17938 and C-peptide secretion was significantly promoted by L. reuteri 6475; although for both hormones, the other L. reuteri strain promoted secretion at p<0.1 (Figure 3C, E). PYY, who secretion was promoted, was not transcriptionally upregulated by either L. reuteri strain. PP (PPY), amylin (IAPP), and insulin (INS) gene counts were below the limit of detection in the RNA-Seq data.
Figure 3.
L. reuteri promotes the secretion of known enteroendocrine-derived intestinal hormones. A) 1) In order to measure the release of intestinal hormones from human intestinal organoids (HIO), L. reuteri conditioned media is generated from mid-log phase cultures of L. reuteri. These cultures are pH neutralized and rendered cell-free. 2) L. reuteri conditioned media is then placed onto NGN3-HIOs plated on transwells that are differentiated but not induced for NGN3 or induced for NGN3. 3) Following an incubation on the HIOs, the supernatant is collected and secreted hormones are measured by ELISA or Luminex assay. Created with BioRender.com. Secreted amylin (B), C-peptide (C), ghrelin (D), GIP (E), PP (F), and PYY (G) measured from uninduced and induced NGN3-HIOs in response to L. reuteri 6475 or 17983 conditioned media. Hormones in B-G were measured on the apical side only of the transwell. In B-G, batches A and B from the RNASeq experiment were pooled so each point on the plot is the result from two organoid batches pooled together. H) Serotonin released from the apical or basolateral side (as indicated) from uninduced and induced NGN3-HIOs in response to L. reuteri 6475 or 17983 conditioned media. In H, shape denotes independent batches of organoids. Only p-values <0.1 are shown with p<0.05 being considered significant. Significance was determined with a Dunnett’s Test.
Interestingly, no genes related to serotonin-metabolism or transporters (TPH1, TPH2, DDC, SLC18A1, SERT) were altered by either L. reuteri strain. Nevertheless, we observed that L. reuteri 6475 and 17938 promote serotonin secretion (Figure 3H). Collectively, these data indicate that L. reuteri regulates numerous gut hormones; however, L. reuteri may upregulate either or both the expression and secretion of intestinal hormones.
L. reuteri affects the transcription and secretion of enterocytic hormones
While the genes in cluster 1 were upregulated by NGN3 induction, those in cluster 5 were downregulated by NGN3 induction (Figure 2). The genes downregulated were for hormones vasopressin (AVP), adipolin (C1QTNF12), luteinizing hormone subunit B (LHB), neurotensin (NTS), and oxytocin (OXT). Neuregulin-4 (NRG4) and tachykinin-3 (TAC3) were unaffected by induction. All these hormone genes were significantly upregulated by L. reuteri 6475, while only LHB and OXT were significantly upregulated by L. reuteri 17938. Interestingly among these hormones, only neurotensin is well established to be produced by the gut epithelium. In mice, neurotensin is observed within villus proximal enteroendocrine L-cells 102,103 and is thought to be produced in L cells only after they have migrated away from crypts and are exposed to increasing levels of BMP4 signaling102.
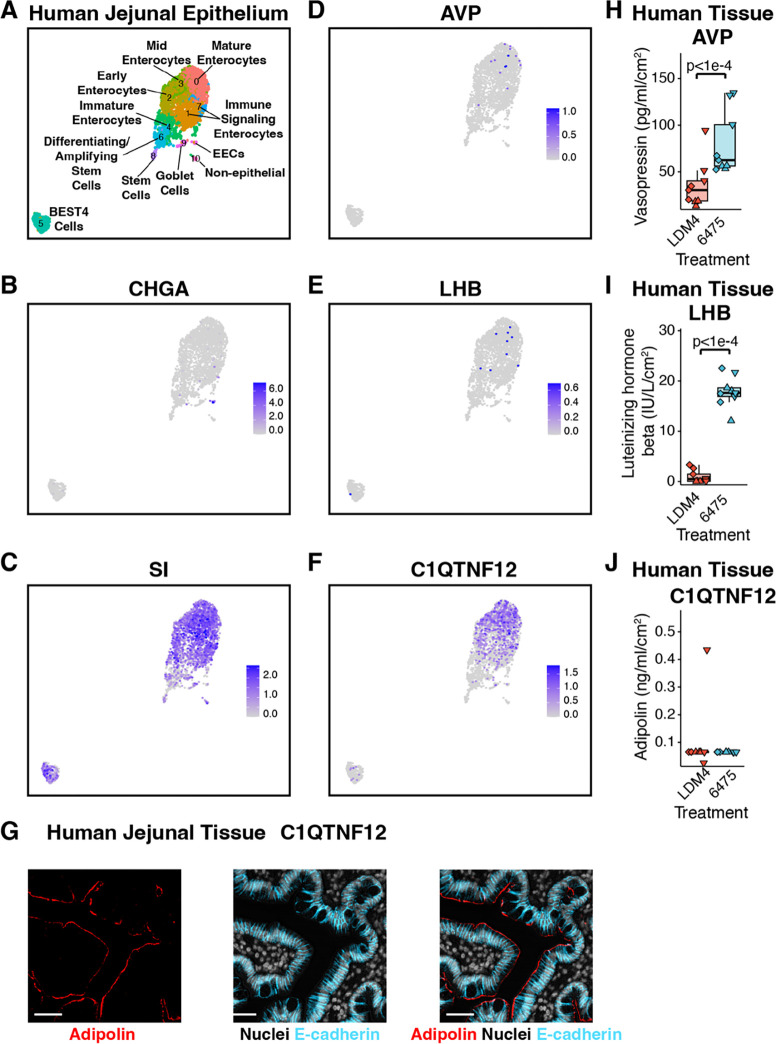
Recently we reported that oxytocin is produced by enterocytes in the small intestinal epithelium and its secretion is promoted by L. reuteri33. To determine if any of these hormones are also produced by enterocytes, we analyzed the adult jejunum single-cell RNA-Seq (scRNA-Seq) data within the Gut Cell Atlas104. While chromogranin A (CHGA) transcription clustered with enteroendocrine cells, transcription of AVP, LHB, and C1QTNF12 (adipolin) clustered similarly to that for sucrose isomatase (SI), a marker of enterocytes (Figure 4A–F). Furthermore, we were able to confirm that C1QTNF12 (adipolin) is produced in enterocytes in the human jejunum (Figure 4G).
Figure 4:
L. reuteri promotes the secretion of enterocytic hormones. A) Gut Cell Atlas annotated UMAP of the adult jejunum (adapted from Danhof et al 2023), highlighting the enteroendocrine marker CHGA (B), the enterocyte marker SI (C), vasopressin (AVP, D), luteinizing hormone subunit beta (LHB, E), and adipolin (C1QTNF12, F). G) Adipolin visualized in human jejunal tissue. Scale bar represents 50 μm. Secretion of H) vasopressin and I) luteinizing hormone subunit beta and J) the lack of secretion of adipolin from whole human jejunal tissue using the method shown in Figure 3A except with ex vivo human jejunal intestinal tissue. Shape represents unique human intestinal donors. Significance was determined using a linear mixed model with p <0.05 considered as significant.
Next we checked if L. reuteri is able to induce the secretion of any of these hormones from whole intestinal tissue as it does for oxytocin33. L. reuteri was able to induce the release of vasopressin and LHB but not adipolin from the human jejunum (Figure 4H–J). Given that AVP and LHB transcription are enriched in epithelial cells in adult gut tissue104 (p = 4.1e-3 for AVP in epithelium across the entire adult intestine, p = 0 for just jejunum; p = 1.0e-5 for LHB in epithelium across the entire adult intestine, p = 0.014 for just jejunum, hypergeometric distribution), the released vasopressin and LHB may originate from the epithelium rather than other regions of the intestinal tissue.
In looking at the functions of the hormones in cluster 5, these hormones have roles in sexual function and behavior, whereas those in cluster 1 have functions mostly in feeding behavior and cardiovascular function. We also noticed that kisspeptin (KISS1), a hormone characterized in the brain with roles in gonad development105, though not differentially regulated by L. reuteri, was expressed in the NGN3-HIOs and downregulated by induction. Like the other hormones in cluster 5, KISS1 appears to be produced in enterocytes (Supplemental Figure 6A). We looked to see if L. reuteri could induce its secretion and found no evidence of L. reuteri mediates release of KISS1 (Supplemental Figure 6B).
Discussion
L. reuteri has been characterized as a beneficial microbe capable of affecting multiple aspects of host physiology within and beyond the gut. These effects are likely to involve host-microbe interactions that initiate at the intestinal epithelial layer. To begin to understand those interactions, here we used an organoid model enhanced in its number of enteroendocrine cells to specifically study interactions between L. reuteri and intestinal hormones. While, microbes have been identified that promote the release or expression of hormones or neuropeptides including GLP-1106–108, PYY107,108, serotonin106,109–112, testosterone26, and oxytocin33, our study here focused on the effect of a single microbe on intestinal hormones using a human intestinal organoid model system. Our results indicate that multiple intestinal hormones are regulated by L. reuteri (Table 4); and moreover, these data point towards there being novel hormones derived from enterocytes in the gut. Specifically, while luteinizing hormone subunit beta was previously observed in the stomach and duodenum113, kisspeptin, adipolin, and vasopressin have not been described as intestinal epithelial hormones.
Table 4:
Summary of L. reuteri’s effects on gut hormones
| Hormone | Proposed or established hormone cell type | Expression | Secretion |
|---|---|---|---|
| Amylin | Enteroendocrine | ND | + (this work) |
| C-peptide | Enteroendocrine | ND | + (this work) |
| CCK | Enteroendocrine | + (this work) | ND |
| Gastrin | Enteroendocrine | + (this work) | ND |
| Ghrelin | Enteroendocrine | + (this work) | NS (this work) |
| GIP | Enteroendocrine | + (this work) | + (this work) |
| Luteinizing hormone, beta subunit | Enterocyte | + (this work) | + (this work)* |
| Motilin | Enteroendocrine | + (this work) | ND |
| Neurotensin | Enteroendocrine | + (this work) | ND |
| NPW | Enteroendocrine | + (this work) | ND |
| NPY | Enteroendocrine | + (this work) | ND |
| Oxytocin | Enterocyte | + (this work) | +33 |
| PYY | Enteroendocrine | NS (this work) | + (this work) |
| Secretin | Enteroendocrine | NS (this work) | +33 |
| Serotonin | Enteroendocrine | NS (this work) | + (this work) |
| Somatostatin | Enteroendocrine | + (this work) | ND |
| Vasopressin | Enterocyte | + (this work) | + (this work)* |
+, upregulated; −, downregulated; ND, not determined; NS, not significant
not confirmed if secretion occurs from epithelial cells
While we found several well-known intestinal hormones are not regulated by L. reuteri (including GLP-1 and pancreatic peptide (PP), we observed that L. reuteri largely transcriptionally upregulates gut hormones. We also found that a smaller set of gut hormones is secreted by L. reuteri. This study was particularly focused on the effect of L. reuteri on hormones of the small intestine, where we postulate L. reuteri may act therapeutically in humans. Hence, these data broadly suggest that L. reuteri can potentially act beneficially via regulation of intestinal hormones. Moreover, our study considered not just a single probiotic strain of L. reuteri but two different commercially used strains. Interestingly, our study failed to observe major differences between the two strains: L. reuteri 17938 appeared to transcriptionally affect HIOs enriched in enteroendocrine cells very similarly to L. reuteri 6475, albeit with a lower magnitude. Furthermore, the select hormones whose secretion we tested were similarly induced by both strains. An unknown experimental condition could be responsible for L. reuteri 17938’s lower effect on the HIO transcripts.
Recently, several new enteric hormones have been described. In addition to the discovery of oxytocin in the intestinal epithelium, famsin114, GDF15115, and cholesin116 have been discovered. A survey of these peptide hormones in the Gut Cell Atlas104 suggests that, in addition to the previously described FGF19, guanylin, and uroguanylin31, these hormones are made in enterocytes rather than enteroendocrine cells. The recognition that enterocytes can produce hormones has opened questions regarding the production of these hormones. Enteroendocrine cell-derived hormones are produced from prohormones that are cleaved to the active hormone by prohormone convertases some of which are exclusively produced in enteroendocrine cells117 and are subsequently secreted from vesicles stored in axon-like structures within the cell118 on stimulation. Hence, are these enterocytic hormones only processed by convertases that are made in enterocytes? Are the hormones stored in vesicles like in enteroendocrine cells? And how and to where are these vesicles released?
The function of these novel enterocytic hormones is additionally waiting to be determined. Interestingly, non-intestinal sources of oxytocin, vasopressin, kisspeptin, and luteinizing hormone have roles in regulating sexual function, and several also function in regulating eating or digestion. Famsin114, GDF15115, and cholesin116 have been characterized with roles related to metabolism and energy regulation. Given the known links between metabolic state and sexual function119, potentially then, intestinal sources of oxytocin, vasopressin, kisspeptin, and luteinizing hormone serve to link metabolic state to sexual function.
We also observed that adipolin is produced in the small intestinal epithelial layer. Adipolin has been observed as present in the small intestinal epithelium presented by the Human Protein Atlas (https://www.proteinatlas.org/ENSG00000184163-C1QTNF12/tissue/small+intestine)120. In adipose tissue, adipolin was characterized as an adipokine that improves glucose tolerance and insulin response and reduces macrophages and proinflammatory immune responses86. In the intestine, it may have similar immune and metabolic functions.
Previously we determined that the hormone secretin is involved in L. reuteri’s release of oxytocin33. However, what L. reuteri makes to promote secretin’s release is currently unknown. Presently, a variety of different microbial metabolites or structures have been shown to promote the release of or are associated with the release of intestinal hormones. These include short chain fatty acids121–123, branched and aromatic amino acids123, indoles124, secondary bile acids125, and microvesicles112. Whether any of these molecules or others produced by L. reuteri are involved in the hormones affected here remains to be determined.
A few limitations of our study design should be mentioned. First, the media conditions of the organoids have been observed to reduce inflammatory responses126. Second, the organoids only represent the epithelial layer so interactions between L. reuteri and the host that depend on immune cells, enteric neurons, or products of the lamina propria or circulation cannot be captured by this assay. Third, the assay was performed using cell-free supernatants with a three-hour exposure. Hence, host responses that require intact structural components of L. reuteri or a different length of exposure are also not represented in this assay. Fourth, the secretion assays were not designed to capture whether L. reuteri suppresses the secretion of hormones, and similarly the transcriptomic data only considers L. reuteri’s effect relative to bacterial growth media. Further follow-up studies will be needed to determine if L. reuteri is able to promote secretion of these hormones under more physiologically relevant conditions.
In conclusion, this work demonstrates that L. reuteri regulates several canonical and novel hormones of the intestinal epithelial layer. These results open exciting investigations regarding how L. reuteri may influence a wide range of aspects of systemic physiology.
Supplementary Material
Acknowledgements:
We would like to thank Susan Venable, Colleen Ardis, Javier Nieto, and the LifeGift donor families for their assistance and support during this project. This project was supported in part by NIH grant DK056338 (Cellular and Molecular Core, Functional Genomics and Microbiome Core, and Gastrointestinal Experimental Model Systems Core), which supports the Texas Medical Center Digestive Diseases Center. This project was supported in part by the Optical Imaging and Vital Microscopy Core at Baylor College of Medicine.
Funding:
U.S. National Library of Medicine [T15 LM007093] (SCD), National Institute of Allergy and Infectious Diseases [F32 AI136404] (HAD), Weston Family Foundation (SCD), BioGaia AB (RAB).
Abbreviations:
- HIO:
human intestinal organoid
- DEG:
differentially expressed gene
Footnotes
COI statement: The authors declare no conflict of interest.
References
- 1.Savino F., Pelle E., Palumeri E., Oggero R. & Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) Versus Simethicone in the Treatment of Infantile Colic: A Prospective Randomized Study. Pediatrics 119, e124–e130 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Nilsson A. G., Sundh D., Bäckhed F. & Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 284, 307–317 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Thomas C. M. et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PloS One 7, e31951 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter J., Britton R. A. & Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. U. S. A. 108 Suppl 1, 4645–4652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y. P., Thibodeaux C. H., Peña J. A., Ferry G. D. & Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm. Bowel Dis. 14, 1068–1083 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Jones S. E. & Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 9, 35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffington S. A. et al. Microbial Reconstitution Reverses Maternal Diet- Induced Social and Synaptic Deficits in Offspring. Cell 165, 1762–1775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sgritta M. et al. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 101, 246–259.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffington S. A. et al. Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell 184, 1740–1756.e16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt L. M. et al. Results of a phase Ib study of SB-121, an investigational probiotic formulation, a randomized controlled trial in participants with autism spectrum disorder. Sci. Rep. 13, 5192 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzone L. et al. Precision microbial intervention improves social behavior but not autism severity: A pilot double-blind randomized placebo-controlled trial. Cell Host Microbe 32, 106–116.e6 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Rosander A., Connolly E. & Roos S. Removal of Antibiotic Resistance Gene-Carrying Plasmids from Lactobacillus reuteri ATCC 55730 and Characterization of the Resulting Daughter Strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 74, 6032–6040 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinler J. K. et al. From Prediction to Function Using Evolutionary Genomics: Human-Specific Ecotypes of Lactobacillus reuteri Have Diverse Probiotic Functions. Genome Biol. Evol. 6, 1772–1789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indrio F. et al. The Effects of Probiotics on Feeding Tolerance, Bowel Habits, and Gastrointestinal Motility in Preterm Newborns. J. Pediatr. 152, 801–806 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Coccorullo P. et al. Lactobacillus reuteri (DSM 17938) in Infants with Functional Chronic Constipation: A Double-Blind, Randomized, Placebo-Controlled Study. J. Pediatr. 157, 598–602 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Miniello V. L. et al. Lactobacillus reuteri modulates cytokines production in exhaled breath condensate of children with atopic dermatitis. J. Pediatr. Gastroenterol. Nutr. 50, 573–576 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Quach D., Parameswaran N., McCabe L. & Britton R. A. Characterizing how probiotic Lactobacillus reuteri 6475 and lactobacillic acid mediate suppression of osteoclast differentiation. Bone Rep. 11, 100227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rios-Arce N. D. et al. Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes. Bone 134, 115269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schepper J. D. et al. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 35, 801–820 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Schepper J. D. et al. Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 34, 681–698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J. et al. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology 156, 3169–3182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Britton R. A. et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 229, 1822–1830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCabe L. R., Irwin R., Schaefer L. & Britton R. A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice: L. reuteri PROMOTES INTESTINE AND BONE HEALTH. J. Cell. Physiol. 228, 1793–1798 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poutahidis T. et al. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLOS ONE 8, e78898 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdman S. & Poutahidis T. Probiotic ‘glow of health’: it’s more than skin deep. Benef. Microbes 5, 109–119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poutahidis T. et al. Probiotic Microbes Sustain Youthful Serum Testosterone Levels and Testicular Size in Aging Mice. PLoS ONE 9, e84877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu Q., Tavella V. J. & Luo X. M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saulnier D. M. et al. Exploring Metabolic Pathway Reconstruction and Genome-Wide Expression Profiling in Lactobacillus reuteri to Define Functional Probiotic Features. PLoS ONE 6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y. et al. Probiotic-Derived Ecto-5’-Nucleotidase Produces Anti-Inflammatory Adenosine Metabolites in Treg-Deficient Scurfy Mice. Probiotics Antimicrob. Proteins 15, 1001–1013 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthington J. J., Reimann F. & Gribble F. M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 11, 3–20 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Bany Bakar R., Reimann F. & Gribble F. M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 20, 784–796 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Chang-Graham A. L. et al. Human Intestinal Enteroids With Inducible Neurogenin-3 Expression as a Novel Model of Gut Hormone Secretion. Cell. Mol. Gastroenterol. Hepatol. 8, 209–229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danhof H. A., Lee J., Thapa A., Britton R. A. & Di Rienzi S. C. Microbial stimulation of oxytocin release from the intestinal epithelium via secretin signaling. Gut Microbes 15, 2256043 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illumina Inc. Sequencing Analysis Software User Guide for Pipeline Version 1.3 and CASAVA Version 1.0 Illumina Inc. (San Diego, CA, USA, 2008). [Google Scholar]
- 35.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anders S., Pyl P. T. & Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge S. X., Son E. W. & Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics 19, 534 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oksanen J. et al. vegan: Community Ecology Package. (2019). [Google Scholar]
- 39.Gu Z., Eils R. & Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (2016). [DOI] [PubMed] [Google Scholar]
- 40.Kassambara A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. (2019). [Google Scholar]
- 41.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 6, 65–70 (1979). [Google Scholar]
- 42.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995). [Google Scholar]
- 44.Zerbino D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fabregat A. et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 46, D649–D655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jassal B. et al. The reactome pathway knowledgebase. Nucleic Acids Res. gkz1031, 1–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mi H. et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 14, 703–721 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stelzer G. et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 54, (2016). [DOI] [PubMed] [Google Scholar]
- 49.Ritchie M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47–e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 51.Smid M. et al. Gene length corrected trimmed mean of M-values (GeTMM) processing of RNA-seq data performs similarly in intersample analyses while improving intrasample comparisons. BMC Bioinformatics 19, 236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma. Oxf. Engl. 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haber A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reaux A., Fournie-Zaluski M. C. & Llorens-Cortes C. Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol. Metab. 12, 157–162 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Szczepańska-Sadowska E. Interaction of vasopressin and angiotensin II in central control of blood pressure and thirst. Regul. Pept. 66, 65–71 (1996). [DOI] [PubMed] [Google Scholar]
- 56.van Unen J. et al. Kinetics of recruitment and allosteric activation of ARHGEF25 isoforms by the heterotrimeric G-protein Gαq. Sci. Rep. 6, 36825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coate K. C., Kliewer S. A. & Mangelsdorf D. J. SnapShot: Hormones of the Gastrointestinal Tract. Cell 159, 1478–1478.e1 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Luyer M. D. et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J. Exp. Med. 202, 1023–1029 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovick T. A. CCK as a modulator of cardiovascular function. J. Chem. Neuroanat. 38, 176–184 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Mace O. J., Tehan B. & Marshall F. Pharmacology and physiology of gastrointestinal enteroendocrine cells. Pharmacol. Res. Perspect. 3, e00155 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutzwiller J.-P. et al. Effect of intravenous human gastrin-releasing peptide on food intake in humans. Gastroenterology 106, 1168–1173 (1994). [DOI] [PubMed] [Google Scholar]
- 62.Russo F. et al. The obestatin/ghrelin ratio and ghrelin genetics in adult celiac patients before and after a gluten-free diet, in irritable bowel syndrome patients and healthy individuals: Eur. J. Gastroenterol. Hepatol. 29, 160–168 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Malik S., McGlone F., Bedrossian D. & Dagher A. Ghrelin Modulates Brain Activity in Areas that Control Appetitive Behavior. Cell Metab. 7, 400–409 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Zhang G. et al. Ghrelin and Cardiovascular Diseases. Curr. Cardiol. Rev. 6, 62–70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H. et al. Gastric neuropeptide W is regulated by meal-related nutrients. Peptides 62, 6–14 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Baker J. R., Cardinal K., Bober C., Taylor M. M. & Samson W. K. Neuropeptide W acts in brain to control prolactin, corticosterone, and growth hormone release. Endocrinology 144, 2816–2821 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Levine A. S., Winsky-Sommerer R., Huitron-Resendiz S., Grace M. K. & de Lecea L. Injection of neuropeptide W into paraventricular nucleus of hypothalamus increases food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1727–1732 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Manfredi-Lozano M., Roa J. & Tena-Sempere M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol. 48, 37–49 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Lim Ramon. Neuropeptide Y in Brain Function. in Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides 524–543 (Springer; US, New York, NY, 2006). [Google Scholar]
- 70.Gribble F. M. & Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu. Rev. Physiol. 78, 277–299 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Carroll R. Endocrine System. in Elsevier’s Integrated Physiology 157–176 (Elsevier Health Sciences, 2007). [Google Scholar]
- 72.Saras J., Grönberg M., Stridsberg M., Oberg K. E. & Janson E. T. Somatostatin induces rapid contraction of neuroendocrine cells. FEBS Lett. 581, 1957–1962 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Serio R. & Zizzo M. G. The multiple roles of dopamine receptor activation in the modulation of gastrointestinal motility and mucosal function. Auton. Neurosci. 244, 103041 (2023). [DOI] [PubMed] [Google Scholar]
- 74.Villarroya F., Cereijo R., Villarroya J. & Giralt M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13, 26–35 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Wang Y., Huang S. & Yu P. Association between circulating neuregulin4 levels and diabetes mellitus: A meta-analysis of observational studies. PLOS ONE 14, e0225705 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Temur M. et al. Increased serum neuregulin 4 levels in women with polycystic ovary syndrome: A case-control study. Ginekol. Pol. 88, 517–522 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Akema T., Praputpittaya C. & Kimura F. Effects of Preoptic Microinjection of Neurotensin on Luteinizing Hormone Secretion in Unanesthetized Ovariectomized Rats with or without Estrogen Priming. Neuroendocrinology 46, 345–349 (1987). [DOI] [PubMed] [Google Scholar]
- 78.Prague J. K. & Dhillo W. S. Neurokinin 3 receptor antagonism – the magic bullet for hot flushes? Climacteric 20, 505–509 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Lim Ramon. Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides. (Springer; US, New York, NY, 2006). [Google Scholar]
- 80.Nielsen S. et al. Aquaporins in the Kidney: From Molecules to Medicine. Physiol. Rev. 82, 205–244 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Robertson G. L. Abnormalities of thirst regulation. Kidney Int. 25, 460–469 (1984). [DOI] [PubMed] [Google Scholar]
- 82.Thornton S. N. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 100, 15–21 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Carroll H. A. & James L. J. Hydration, Arginine Vasopressin, and Glucoregulatory Health in Humans: A Critical Perspective. Nutrients 11, 1201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim Ramon. Oxytocin and Vasopressin: Genetics and Behavioral Implications. in Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides 574–607 (Springer; US, New York, NY, 2006). [Google Scholar]
- 85.Walum H. & Young L. J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 19, 643–654 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enomoto T. et al. Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J. Biol. Chem. 286, 34552–34558 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barbe A. et al. Adipolin (C1QTNF12) is a new adipokine in female reproduction: expression and function in porcine granulosa cells. Reprod. Camb. Engl. 167, e230272 (2024). [DOI] [PubMed] [Google Scholar]
- 88.Lee S. L. et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273, 1219–1221 (1996). [DOI] [PubMed] [Google Scholar]
- 89.Adriana Lofrano-Porto et al. Luteinizing Hormone Beta Mutation and Hypogonadism in Men and Women. N. Engl. J. Med. 357, 897–904 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Jacob S. et al. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci. Lett. 417, 6–9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welch M. G., Margolis K. G., Li Z. & Gershon M. D. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am. J. Physiol. - Gastrointest. Liver Physiol. 307, G848–G862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Afroze S. et al. The physiological roles of secretin and its receptor. Ann. Transl. Med. 1, 29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y., Bond J. & Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. U. S. A. 100, 2237–2242 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hwang S. J. et al. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J. Physiol. 590, 1957–1972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma J. et al. Glycogen metabolism regulates macrophage-mediated acute inflammatory responses. Nat. Commun. 11, 1769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bates D., Mächler M., Bolker B. & Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 97.Lenth R. V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 180 https://CRAN.R-project.org/package=emmeans, (2022). [Google Scholar]
- 98.Allaire J. M. et al. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 39, 677–696 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Migone T.-S. et al. TL1A Is a TNF-like Ligand for DR3 and TR6/DcR3 and Functions as a T Cell Costimulator. Immunity 16, 479–492 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Dheer R. et al. Intestinal Epithelial Toll-Like Receptor 4 Signaling Affects Epithelial Function and Colonic Microbiota and Promotes a Risk for Transmissible Colitis. Infect. Immun. 84, 798–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim S.-H. et al. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc. Natl. Acad. Sci. U. S. A. 97, 1190–1195 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beumer J. et al. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signaling gradient. Nat. Cell Biol. 20, 909–916 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roth K. A., Kim S. & Gordon J. I. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am. J. Physiol. 263, G174–180 (1992). [DOI] [PubMed] [Google Scholar]
- 104.Elmentaite R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie Q. et al. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 13, 925206 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomaro-Duchesneau C. et al. Discovery of a bacterial peptide as a modulator of GLP-1 and metabolic disease. Sci. Rep. 10, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breton J. et al. Gut Commensal E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. Cell Metab. 23, 324–334 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Rabiei S., Hedayati M., Rashidkhani B., Saadat N. & Shakerhossini R. The Effects of Synbiotic Supplementation on Body Mass Index, Metabolic and Inflammatory Biomarkers, and Appetite in Patients with Metabolic Syndrome: A Triple-Blind Randomized Controlled Trial. J. Diet. Suppl. 16, 294–306 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Ye L. et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 29, 179–196.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Vadder F. et al. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 1–13 (2014) doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 111.Yano J. M. et al. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yaghoubfar R. et al. The impact of Akkermansia muciniphila and its extracellular vesicles in the regulation of serotonergic gene expression in a small intestine of mice. Anaerobe 83, 102786 (2023). [DOI] [PubMed] [Google Scholar]
- 113.Busslinger G. A. et al. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep. 34, 108819 (2021). [DOI] [PubMed] [Google Scholar]
- 114.Long A. et al. Famsin, a novel gut-secreted hormone, contributes to metabolic adaptations to fasting via binding to its receptor OLFR796. Cell Res. 33, 273–287 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coll A. P. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu X. et al. A gut-derived hormone regulates cholesterol metabolism. Cell 187, 1685–1700.e18 (2024). [DOI] [PubMed] [Google Scholar]
- 117.Dhanvantari S., Seidah N. G. & Brubaker P. L. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol. Endocrinol. 10, 342–355 (1996). [DOI] [PubMed] [Google Scholar]
- 118.Bohórquez D. V. et al. An Enteroendocrine Cell – Enteric Glia Connection Revealed by 3D Electron Microscopy. PLoS ONE 9, e89881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Izzi-Engbeaya C. & Dhillo W. S. Gut hormones and reproduction. Ann. Endocrinol. 83, 254–257 (2022). [DOI] [PubMed] [Google Scholar]
- 120.Uhlén M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Kimura I. et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 4, 1829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chambers E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lund M. L. et al. Enterochromaffin 5-HT cells – A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 11, 70–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chimerel C. et al. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 9, 1202–1208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Q. et al. Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes 15, 2274124 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruan W. et al. Enhancing responsiveness of human jejunal enteroids to host and microbial stimuli. J. Physiol. 598, 3085–3105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq reads are available at NCBI GEO at https://www.ncbi.nlm.nih.gov/geo/ accession number GSE138350 and GSE268681. Scripts for plots and data are available at https://github.com/sdirienzi/Lreuteri_HIORNASeq. An interactive ShinyApp displaying the RNASeq data can be found here: https://sdirienzi.shinyapps.io/LreuHIORNASeq/.